Autolus: CAR-T Therapy Looks Promising
Summary
- Autolus is a UK-based CAR-T therapy developer with a lead program, obecabtagene autoleucel, that has met the endpoint in a pivotal trial for adult acute lymphoblastic leukemia.
- Obecabtagene autoleucel has a faster off-rate than other CAR-T therapies, potentially leading to increased efficacy and safety.
- The trial results showed a 70% complete remission rate and a manageable safety profile, with 61% of responders still in remission at 9.5 months of follow-up.
- Looking for more investing ideas like this one? Get them exclusively at The Total Pharma Tracker. Learn More »
Pgiam/iStock via Getty Images
Autolus (NASDAQ:AUTL) is a CAR-T therapy developer with in-house manufacturing, multiple collaborations and a lead program that just met the endpoint in a pivotal trial in ALL. The company is UK-based. Its programs are described thus on Seeking Alpha:
The company’s clinical-stage programs include obecabtagene autoleucel (AUTO1), a CD19-targeting programmed T cell investigational therapy that is in Phase 1b/2 clinical trial for the treatment of adult ALL; AUTO1/22, which is in a Phase 1 clinical trial in pediatric patients with relapsed or refractory ALL; AUTO4, a programmed T cell investigational therapy for the treatment of peripheral T-cell lymphoma targeting TRBC1 and TRBC2; AUTO6NG, a programmed T cell investigational therapy, which is in preclinical trial targeting GD2 in development for the treatment of neuroblastoma; and AUTO8, a product candidate that is in a Phase I clinical trial for multiple myeloma. It also focuses on developing AUTO5, a preclinical TRBC2 programmed T cell product candidate for the treatment of peripheral T-cell lymphoma.
Lead program is obecabtagene autoleucel, a CD19 directed autologous CAR-T. It has been tested in R/R pediatric and adult B-ALL3 , as well as in other B-cell malignancies. The asset has met the primary endpoint in a pivotal study called FELIX in relapsed/ refractory adult acute lymphoblastic leukemia (ALL).
Obe-cel, the company says, is a CD19 binder that also has a fast off-rate. That means, while it binds to CD19, it also dissociates from the linkage quickly and has a shorter half life of interaction. To be specific, while Obe-cel takes 9.8 seconds for the binding and unbinding, Kymriah takes 21 minutes. Potentially, this has a positive impact on both efficacy and safety. Since these bindings are lossless, Obe-cel can quickly move on from one antibody to another, increasing proliferation, immune response and thus, effectiveness of a relatively lower dose of therapy. That, and the reduced persistence of linkage, also produces a better safety profile by avoiding over-activation of CAR T cells and reducing toxicities.
At ASCO and EHA 2023, Autolus presented data from FELIX. Here’s the link to the ASCO data. Here’s most of the presentation:
Methods: FELIX is an open-label, multi-centre, global, single-arm Phase Ib/II study enrolling r/r B-ALL patients with morphological disease (≥5% BM blasts), measurable residual disease (MRD) (≥0.1% to < 5% BM blasts), or isolated extramedullary disease (Cohorts A, B and C). CAR-T products were generated using an automated closed process from fresh leukapheresate. Patients underwent bridging therapy as necessary and lymphodepletion with fludarabine (4x30mg/m2) and cyclophosphamide (2x500mg/m2). Patients received a target dose of 410E+6 CAR T cells as a split dose on Day 1 and Day 10. The dosing schedule is based on the % BM blasts performed locally prior to the pre-conditioning. Primary endpoint was overall remission rate (ORR) defined as proportion of patients achieving CR/CRi by central assessment. Results: As of 9th September 2022, a pre-specified interim analysis was conducted based on the first 50 patients infused in Cohort A who have been followed for 3 months or discontinued before month 3. The median age was 50 years (range 20-81), 22% had Ph+ B-ALL. The median number of prior lines of treatment was 2 (range 1-5), 42% underwent prior transplant. At screening, patients had a median of 55% BM blasts (range 6-96%) and 26% had EMD. The geometric mean of peak CAR expansion was 126147.6 copies/ug genomic DNA. Persistence was ongoing in majority of responders at last follow-up. Based on central assessment, the CR/CRi was 70% [95% CI: 55%, 82%] (p-value < 0.0001). As of 9th September 2022, a total of 92 patients received obe-cel and were evaluable for safety. 63% developed any grade CRS (3% Grade ≥3) at a median of 9 days post-infusion and a median duration of 5 days. Any grade ICANS was observed in 23% (8% Grade ≥3) at a median of 15 days post-infusion and of median duration 8 days. Other common Grade≥3 adverse events regardless of causality were febrile neutropenia (25%) and anaemia (20%). Conclusions: The pre-specified interim analysis of the FELIX study demonstrated that obe-cel for adult r/r B-ALL is safe with low rates of Grade >3 CRS and/or ICANS, even in patients with high burden disease. Obe-cel is effective with high CR/CRi rates and ongoing CAR T persistence in the majority of responders. The trial has completed dosing of all patients in Cohort A. Additional data and longer follow up will be reported at the conference.
Thus, there was a 70% CR rate and a manageable safety profile in treatment-experienced patients who had undergone multiple lines of therapy. Duration of response, a key positive criteria for an expensive, late line therapy, was also good, with 61% of responders under remission at 9.5 months of follow up, needing no other cancer therapy.
In its early years, AUTL went through a few issues that delayed the stock’s progress. In 2019, for example, it let go of a lead program because the competitive space became crowded, and that particular program just couldn’t compete. In the same year, it faced manufacturing delays because a UK-based plant where it had leased space (or capabilities) could not deliver. It faced funding issues because some of its funding was tied to Neil Woodward’s ventures, which were facing existential threats at that time.
The present obe-cel data shows that the company has put these things back, and has arrived. While Gilead’s CD19 directed autologous CAR-T therapy Tecartus is already approved for ALL, AUTL’s claimed differentiation is a better safety profile and reduced T-cell exhaustion. As the company points out:
Current T cell therapies for adult patients are Blincyto® and TecartusTM – Both therapies are highly active, but frequently followed by subsequent treatments (e.g. alloSCT)
– Blincyto®: favourable safety profile, few patients experiencing severe CRS and ICANS, but limitations on convenience - continuous i.v. infusion during 4-week treatment cycles
– Tecartus™: more challenging to manage - induces elevated levels of severe CRS, a high levels of severe ICANS, and requires vasopressors for many patients
Thus, each of these two products has limitations which obe-cel addresses. Here’s another safety comparison chart versus SoC:
obe-cel vs SoC (autolus website)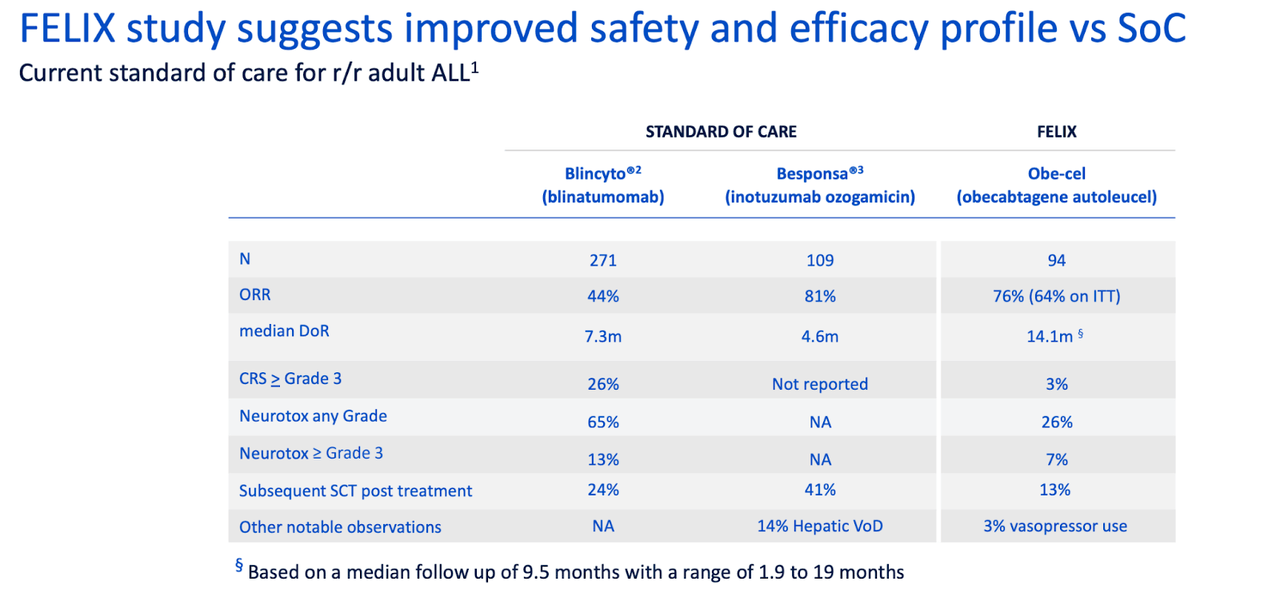
Obe-cel’s only problem is that it is open label and single arm, but autologous CAR-T therapies have that problem because of the difficulty of manufacturing, which precludes a comparator arm. The company is now planning a BLA by the end of the year.
Financials
AUTL has a market cap of $531mn and a cash balance of $308mn. Research and development expenses decreased by $1.5 million to $36.7 million for the three months ended June 30, 2023, while general and administrative expenses increased by $2.8 million to $11.1 million. At that rate, the company has a cash runway of some 6-7 quarters.
PE/VC firms and institutions hold most of AUTL. Key holders are Syncona, Blackstone, and Qatar Investments.
Bottomline
AUTL seems attractive right now, with a long cash runway and a superior product candidate with near term catalysts. I would go for a pilot position.
About the TPT service
Thanks for reading. At the Total Pharma Tracker, we offer the following:-

Our Android app and website features a set of tools for DIY investors, including a work-in-progress software where you can enter any ticker and get extensive curated research material.
For investors requiring hands-on support, our in-house experts go through our tools and find the best investible stocks, complete with buy/sell strategies and alerts.
Sign up now for our free trial, request access to our tools, and find out, at no cost to you, what we can do for you.
This article was written by
Dr Dutta is a retired veterinary surgeon. He has over 40 years experience in the industry. Dr Maiya is a well-known oncologist who has 30 years in the medical field, including as Medical Director of various healthcare institutions. Both doctors are also avid private investors. They are assisted by a number of finance professionals in developing this service.
If you want to check out our service, go here - https://seekingalpha.com/author/avisol-capital-partners/research
Disclaimer - we are not investment advisors.
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Seeking Alpha's Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.


