Minerva Neurosciences: There's Probably Time For One More Rally

Summary
- Minerva Neurosciences has raised $20M through a private placement at a premium to market prices.
- Boehringer Ingelheim participated in the private placement. Boehringer is itself developing a drug for schizophrenia, albeit for cognitive impairments associated with schizophrenia.
- NERV has six months until the PDUFA goal date for its roluperidone application but used only $4M in cash in operating activities in Q2, so cash isn't currently a concern.

Sarah Silbiger
On May 1, 2023, Minerva Neurosciences (NASDAQ:NERV) issued a press release noting the US FDA had filed its New Drug Application (NDA) for roluperidone for negative symptoms in schizophrenia. NERV had to use a dispute resolution process to get the FDA to file that NDA, having initially issued NERV with a refusal to file letter. I wrote about NERV that month, rating it a buy as even though I couldn't predict that the FDA would approve roluperidone, there was some chance of approval and the name was trading at a pretty cheap valuation. This article looks at a key development since and the recent earnings.
Interest from Big Pharma
On June 28, NERV announced it had raised $20M using a private placement at a premium to the market. NERV sold 1.425M shares of its common stock at $10 per share and 575,575 shares worth of pre-funded warrants at $9.99. The buyers were privately held Boehringer Ingelheim (BI), and Federated Hermes Kaufmann Funds. Part of the terms of the private investment in public equity (PIPE) were that BI would get to designate someone to attend all meetings of NERV's board of directors, in a non-voting capacity. BI's contribution to the $20M invested was $15M in the form of 1,275,000 shares and 225,225 pre-funded warrants.
Looking at BI's CNS pipeline it is pretty obvious where their interest lies. BI's Iclepertin is an inhibitor of glycine transporter 1 (GlyT1), currently in development for the treatment of cognitive impairments associated with schizophrenia (CIAS). Iclepertin has already demonstrated improvement of cognition in schizophrenia patients in a phase 2 trial run during 2018-2019, and is currently in several studies as part of a phase 3 program. The three CONNEX studies currently list identical features in terms of enrolment dates, primary and secondary endpoints. It will be worth keeping an eye on these studies for updates as CONNEX-3 has a slightly different title but ends up using the same endpoint.
Table 1: Overview of key efficacy studies in BI's phase 3 program of iclepertin. MCCB refers to the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) consensus cognitive battery (MCCB). A long term extension study to collect additional safety data is also underway.
| Study | ClinicalTrials.gov Identifier | Estimated Enrollment | Estimated Primary Completion Date | Primary Outcome |
| Clinical Trial of Iclepertin Effect on Cognition and Functional Capacity in Schizophrenia (CONNEX-1) | NCT04846868 | 586 | January 3, 2025 | Change from baseline in composite T-Score of the MCCB after 26 weeks |
| Clinical Trial of Iclepertin Effect on Cognition and Functional Capacity in Schizophrenia (CONNEX-2) | NCT04846881 | 586 | January 3, 2025 | Change from baseline in composite T-Score of the MCCB after 26 weeks |
| CONNEX-3: A Study to Test Whether Iclepertin Improves Learning and Memory in People with Schizophrenia | NCT04860830 | 586 | January 3, 2025 | Change from baseline in composite T-Score of the MCCB after 26 weeks |
Much like negative symptoms of schizophrenia, cognitive symptoms of schizophrenia are not well addressed by current medications, so both BI and NERV are going after the untapped side of the schizophrenia market.
Of course the presence of the BI investment doesn't mean NERV's roluperidone NDA will be accepted. Indeed NERV might find it receives a complete response letter (CRL) when the Prescription Drug User Fee Act (PDUFA) goal date February 26, 2024, comes around. Perhaps the main thing NERV will achieve with a CRL is a clearer regulatory path to market, that they can hold the FDA to.
Financial Overview
NERV reported a net loss of $6.2M for Q2'23, with R&D expense of $1.9M, $0.3M of which was from non-cash stock compensation and G&A expense of $2.6M, of which $0.3M was from non-cash compensation. Net cash used in operating activities was just $4M in Q2'23, meaning NERV can stay afloat for a while at the current rate, having ended Q2 with $51.9M in cash, cash equivalents and restricted cash. Of course NERV isn't going to go on for ten or more quarters at the current rate, the company is in a holding period as it awaits a decision from the FDA and costs could kick up closer to the PDUFA goal date. NERV could make new hires to prepare for a potential launch, or engage in other potential pre-launch activities which could increase cash burn. In any case, I don't see cash as a concern near-term. NERV needs more money if it wants to launch roluperidone, but it could raise some prior to a potential approval, if the stock trades up, or following any approval.
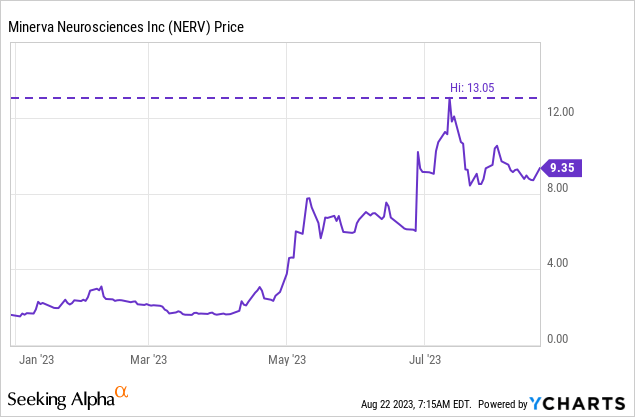
As of July 27, 2023, there were 6,993,406 shares of NERV's common stock outstanding corresponding to a market cap of $65.4M ($9.35 per share, the closing price on Monday, August 21, 2023).
Conclusions and Risks
At the time of writing, NERV sits below the $10 per share price the PIPE was conducted at. Further, the relatively low market cap still doesn't take in much expectation of approval for NERV. As such, despite the fact that NERV is trading well above the level it was trading at when I wrote my previous article, the bull case has been strengthened in two ways. Firstly, NERV has brought in cash, and done so with a raise above market prices, and secondly, the interest of BI in NERV raises the possibility of further investment. I think there is still time for one more long before the PDUFA date, and so I rate NERV a buy. A rally back to the recent high of $13.05 from Monday's closing price of $9.35 would represent a 39.6% gain, even a rally to $10 represents a 7% gain, and I wouldn't be surprised if the name trades to that level within a couple of months.
One of the major risks to any long in NERV at this stage is simply that the company receives early notice that their NDA has not been approved. It might not take the FDA until the PDUFA goal date to make a decision.
Even prior to a decision on the NDA, an advisory committee meeting, if held, could come with negative comments from the advisory committee, including voting against recommending the drug for approval. While the FDA previously said it is not planning to hold an advisory committee, that could change.
We received confirmation on the 27th of April from the FDA that our NDA was filed and on the 8th of May we received confirmation that the review will proceed on a standard review time line with a PDUFA goal date of February 26, 2024. At this time, the FDA stated that it is not planning to hold an advisory committee.
Dr Remy Luthringer, Executive Chairman and CEO of NERV, May 15, 2023.
Lastly NERV might take advantage of any elevated trading of the share price to raise more funds, perhaps at a less favorable price, prior to the PDUFA goal date, causing the stock to trade down.
Editor's Note: This article covers one or more microcap stocks. Please be aware of the risks associated with these stocks.
This article was written by
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Seeking Alpha's Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.
