Pyxis Oncology: A High-Stakes Gambit In The Battle Against Solid Tumors

Summary
- Pyxis Oncology is developing next-generation therapeutics, including antibody-drug conjugates and immuno-oncology programs, to target solid tumors resistant to current treatments.
- PYXS's lead product candidates, PYX-201 and PYX-106, are currently in Phase 1 clinical trials, with promising preclinical results and unique targeting mechanisms.
- Pyxis Oncology presents a 'Buy' opportunity for high-risk-tolerant investors, but investors should monitor the progress of ongoing Phase 1 trials and the competitive landscape.
Wirestock/iStock via Getty Images
Introduction
Pyxis Oncology (NASDAQ:PYXS) is a clinical stage company founded in 2018, specializing in defeating hard-to-treat cancers. They are developing next-generation therapeutics, including antibody-drug conjugates (ADCs) and immuno-oncology (IO) programs, to target solid tumors resistant to current treatments. Their innovative approaches aim to directly kill tumor cells and address cancer's underlying mechanisms for uncontrolled growth and immune evasion. Pyxis Oncology's goal is to provide effective mono and combination therapies, offering new options for patients with challenging cancers.
Recent developments: Pyxis Oncology had acquired Apexigen in a $16M all-stock deal, granting Pyxis access to Apexigen's potential cancer treatments. After the acquisition, Apexigen had become a wholly owned unit of Pyxis, and Pyxis's then-current shareholders held 90% of the merged company.
The following article explores Pyxis Oncology's financials, innovative cancer therapies, and investment prospects, highlighting potential risks for investors in this clinical-stage, microcap company.
Q1 2023 Financials
Let's first review their most recent financial report. As of March 31, 2022, the company had cash and cash equivalents, including restricted cash, and short-term investments totaling $150.8 million. This amount is expected to support operations until the first half of 2025. The cash balance reflects an $8 million payment made to Pfizer, Inc. during the first quarter for expanding the license agreement for Pyxis Oncology's Flexible Antibody Conjugation Technology [FACT] platform. Research and development expenses for the quarter were $11.9 million, down from $20.1 million in the same period last year. General and administrative expenses decreased from $11.3 million in Q1 2022 to $9.1 million in Q1 2023. The net loss for the quarter was $19.2 million, including $4.9 million in non-cash stock-based compensation expense.
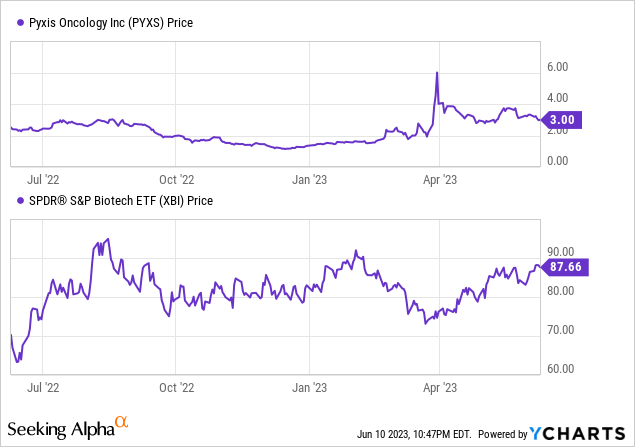
Per Seeking Alpha, Pyxis Oncology's capital structure consists of a market capitalization of $97.51 million, total debt of $20.25 million, cash holdings of $149.35 million, and no other specified assets or liabilities. The enterprise value is reported as -$31.58 million.
Promising ADC and IO Candidates: PYX-201 and PYX-106 in Solid Tumor Cancer Treatment
Pyxis Oncology's leading ADC product candidate, PYX-201, represents a groundbreaking approach to treating numerous solid tumor cancers, such as NSCLC, breast, ovarian, thyroid, PDAC, STS, HCC, and kidney cancer. The candidate drug, licensed from Pfizer, has made progress into Phase 1 clinical trials. PYX-201 specifically targets the Fibronectin EDB protein, often overexpressed in many cancer types but scarce in healthy tissues. Encouraging preclinical results have demonstrated strong anti-tumor activity and immunogenic cell death in both NSCLC and breast cancer mouse models.
Central to PYX-201's therapeutic potential is its ability to directly eradicate tumor cells, modify the tumor microenvironment, and stimulate an anti-tumor immune response. In vitro and in vivo studies have demonstrated the drug's efficiency in reducing tumor burden and inciting an immune response. Furthermore, its compatibility with checkpoint therapy suggests potential for combination treatments for heightened efficacy. Toxicology studies in the preclinical stage showed the drug was well-tolerated, with reversible toxicities and no significant changes in body weight or food intake.
The promising early results and PYX-201's unique ability to target the overexpressed EDB in many hard-to-treat cancers signal high potential for the investigational drug. The ultimate success of this drug, however, will largely hinge on the results of ongoing and future clinical trials. If successful, PYX-201 could present a novel therapeutic option for patients with various solid tumors, particularly those who have failed to respond to standard treatment options. This drug could also potentially be combined with other treatments to further enhance cancer treatment outcomes.
In parallel, PYX-106, the principal IO product candidate, is an innovative antibody developed to counteract T-cell suppression by specifically targeting Siglec-15, a protein abundant in tumor microenvironments (TME). Overexpression of Siglec-15 in tumor cells and M2 macrophages across various cancer types amplifies their immunosuppressive effects, thereby impairing anti-tumor immune responses. Unlike the PD-L1 pathway, Siglec-15 operates independently, marking it a promising therapeutic target, especially for patients who show minimal response to PD-1/PD-L1 targeted therapies. PYX-106 functions by binding to and blocking Siglec-15 activity, thereby augmenting immune cell-mediated destruction of tumor cells. This promising candidate also commenced Phase 1 clinical trials in 2023.
The unique mode of action of PYX-106, targeting Siglec-15-a protein abundant in TME and crucial for immune regulation-offers a novel approach to fighting solid tumors. Especially for patients who show resistance to PD-1/PD-L1 targeted therapies, this approach could offer new hope. Nonetheless, the ultimate success of PYX-106 is uncertain until it's thoroughly evaluated in clinical trials for safety, efficacy, and pharmacological properties.
Taking a look around, their ADCs and immunotherapies are likely to confront significant competition from alternatives such as CAR-T therapies, bispecific antibodies, and small molecules. They're also facing specific target competition, such as EDB from Philogen and Siglec-15 (NC318) from Nextcure. However, Nextcure recently announced the cessation of NC318 development due to disappointing efficacy, despite its safety. This failure could potentially cast a negative shadow on Siglec-15 as a target.
My Analysis & Recommendation
The promising preclinical results of Pyxis Oncology's ADC and IO programs, coupled with its unique targeting mechanisms and the acquisition of potential cancer treatments from Apexigen, make it a compelling prospect for investors focusing on oncology therapeutics. The company's substantial cash and cash equivalents offer a solid financial base, ensuring resources for clinical trials until 2025. However, given the company's sub-zero enterprise value, it appears investors are currently not attributing any value to the company's potential. This assessment seems unjust.
However, the success of Pyxis Oncology as an investment prospect hinges largely on its lead product candidates, PYX-201 and PYX-106, both of which are currently in Phase 1 clinical trials. PYX-201's unique mechanism of action and promising preclinical results suggest a high potential for success in treating various solid tumor cancers. However, the uncertainty of clinical trials and their subsequent results remains a significant risk.
Similarly, PYX-106's mechanism targeting Siglec-15, independent of the PD-L1 pathway, offers a new approach to immunotherapy, especially for patients who are less responsive to existing therapies. But again, its safety, efficacy, and pharmacological properties are still under investigation, with results expected only by late 2023.
Further, while the recent failure of Nextcure's Siglec-15 targeting program does raise questions about the validity of this target, it may also present an opportunity for Pyxis Oncology to learn from their shortcomings and innovate accordingly.
Looking ahead, one of the challenges for Pyxis will be the ability to stand out in a competitive market that includes not just other ADCs and IO therapies but also newer technologies like CAR-T therapies, bispecific antibodies, and small molecules. Competitive threats can lead to pricing pressure and decreased market share if the company is not able to differentiate its products effectively or demonstrate superior outcomes.
Given the clinical stage of its drug candidates and the inherent risks in drug development, Pyxis Oncology may not be suitable for risk-averse investors. However, for those comfortable with the higher risk and volatility often associated with early-stage biotech investments, the potential upside can be significant if the company's drug candidates prove successful.
In summary, Pyxis Oncology presents a speculative 'Buy' opportunity for high-risk-tolerant investors willing to bank on the potential success of the company's novel cancer therapeutics. But investors should monitor the progress of the ongoing Phase 1 trials and the competitive landscape to reassess the investment thesis as necessary. For more conservative investors, Pyxis Oncology may be a company to watch, pending the outcomes of its ongoing clinical trials.
Risks to Thesis
When the facts change, I change my mind.
The key risks to my 'Buy' recommendation for Pyxis Oncology are as follows:
Clinical Trial Risk: PYX-201 and PYX-106 are currently in Phase 1 clinical trials. While they have shown promise in preclinical trials, there is always a significant risk that they may fail in later-stage clinical trials due to unforeseen safety concerns, lack of efficacy, or other reasons. The failure of either candidate in clinical trials could significantly impact the company's share price and overall business strategy.
Regulatory Risk: Even if the company's drug candidates successfully pass clinical trials, there is no guarantee that they will receive regulatory approval from authorities such as the FDA. Any negative regulatory decisions could lead to substantial financial and operational impact on Pyxis.
Competition Risk: Pyxis operates in a highly competitive space with numerous companies developing similar therapies, including ADCs, IO therapies, and other modalities like CAR-T therapies, bispecific antibodies, and small molecules. Competition could impact Pyxis's ability to carve out a niche for itself in the market, affecting the company's potential for sales and growth.
Intellectual Property Risk: Pyxis's success largely depends on its ability to protect its intellectual property, especially concerning its novel therapies. Any failure to secure or maintain strong patent protection could expose the company to competitive threats.
Financial Risk: Although Pyxis has a healthy cash balance that can support operations until 2025, it continues to operate at a loss. There is a risk that the company may need to raise additional capital in the future, which could dilute current shareholders' equity.
Editor's Note: This article covers one or more microcap stocks. Please be aware of the risks associated with these stocks.
This article was written by
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
This article is intended to provide informational content only and should not be construed as personalized investment advice with regard to "Buy/Sell/Hold/Short/Long" recommendations. Any predictions made in this article regarding clinical, regulatory, and market outcomes are the author's opinions and are based on probabilities, not certainties. While the information provided aims to be factual, errors may occur, and readers should verify the information for themselves. Investing in biotech is highly volatile, risky, and speculative, so readers should conduct their own research and consider their financial situation before making any investment decisions. The author cannot be held responsible for any financial losses resulting from reliance on the information presented in this article.
Seeking Alpha's Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.
