Evaluating Brainstorm's 'Hail Mary' For ALS Treatment

Summary
- Brainstorm Cell Therapeutics faces uncertainty in the approval process for its NurOwn treatment for neurodegenerative diseases, with a pivotal ADCOM meeting scheduled for September 27, 2023.
- The company's corrected data from Phase 3 trials show promising results in certain subgroups, but the lack of statistically significant results across the broader trial population presents challenges for approval.
- Brainstorm's financials reveal a decreasing cash balance, which may require the company to seek additional capital soon, adding to the investment risk profile.
koto_feja
Introduction
Brainstorm Cell Therapeutics (NASDAQ:BCLI) is a clinical-stage biotechnology company focused on the development and delivery of advanced autologous cell therapies aimed at neurodegenerative diseases. The firm's unique cell therapy platform, NurOwn, fosters the growth of autologous bone marrow-derived mesenchymal stem cells to produce an abundance of neurotrophic factors. These elements contribute to regulating neuroinflammatory and neurodegenerative processes, promoting neuron survival, and enhancing neurological functionality. Diseases targeted include Amyotrophic Lateral Sclerosis (ALS), Progressive Multiple Sclerosis (PMS), and Alzheimer’s disease (AD), among other neurodegenerative conditions.
In 2022, the company applied for a Biologics License (BLA) for NurOwn, intended for ALS treatment, but encountered a setback when the FDA issued a Refuse To File (RTF) letter due to questions about the clinical trial's efficacy and Chemistry, Manufacturing, and Controls [CMC] processes. In response, Brainstorm held a Type A meeting to address these concerns and subsequently requested to 'File over Protest,' which opened a quicker route to an Advisory Committee [ADCOM] meeting now planned for September 27, 2023.
Following these steps, Brainstorm updated and resubmitted their BLA in March 2023. At the present moment, the FDA is in the process of actively examining the BLA for NurOwn with an action date of December 8, 2023.
This article explores Brainstorm's journey with its cell therapy product, NurOwn, focusing on its FDA review process, financial standing, and the implications for investors amid the ongoing regulatory and clinical uncertainties.
Q1 2023 Financials
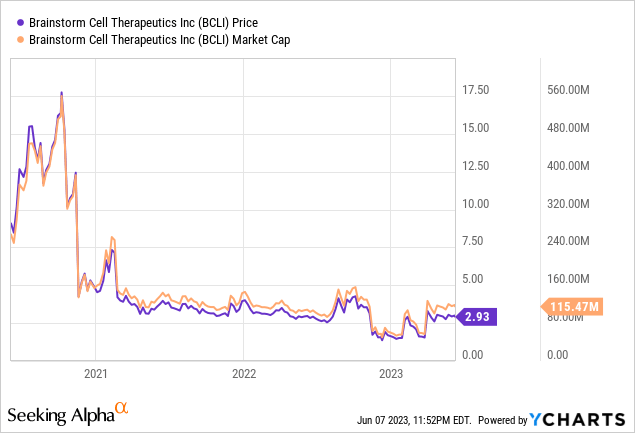
Let's first review the company's most recent financial report. As of March 31, 2023, Brainstorm's cash, cash equivalents, and short-term bank deposits amounted to approximately $2.2 million, down from $3 million at the close of December 2022. The company's research and development expenses for the first quarter of 2023 rose slightly to around $2.9 million from $2.6 million in the same period of 2022. In contrast, general and administrative expenses decreased to about $2.2 million from $2.9 million year over year. Overall, Brainstorm experienced a net loss of about $5.1 million in Q1 2023, a slight improvement from the $5.4 million net loss in Q1 2022. The net loss per share also reduced marginally, from $0.15 in Q1 2022 to $0.14 in Q1 2023.
NurOwn Faces Uncertain Approval Prospects in ALS Treatment Journey
The development journey of NurOwn has progressed through a series of clinical trials across prominent U.S. medical centers and the Hadassah Medical Center in Jerusalem, Israel. Initial trials showed promising signs regarding its tolerability and preliminary effectiveness, setting the stage for more rigorous testing.
In a subsequent Phase 2 study conducted in the U.S., NurOwn underwent a double-blind, placebo-controlled trial involving 48 patients. The results not only reinforced the treatment's tolerability but also revealed clinically meaningful benefits. Most notably, patients who received NurOwn showed higher response rates in the ALS functional rating scale over 24 weeks.
Following this, Phase 3 trials were launched with the aim of generating supportive data for a BLA in the U.S. These trials focused on rapidly progressing ALS patients and maintained the consistent finding that NurOwn was well-tolerated. However, they did not produce statistically significant results. Despite this, the FDA allowed the company to proceed with their BLA submission, suggesting room for further exploration.
Significant updates regarding the Phase 3 trial data arrived when the company rectified some initial data. The corrected data from their Phase 3 clinical trial strengthened their original conclusions and indicated a statistically significant treatment difference in a critical secondary endpoint. Additionally, the corrected analysis highlighted considerable benefits for all subgroups with ALSFRS-R baseline scores between 26 and 35 post-treatment with NurOwn.
This data correction shed new light on the Phase 3 trial results of NurOwn. While initial data released in December 2021 did not show statistically significant results, a correction in August 2022 introduced a significant pivot. In the adjusted data, a significant treatment difference became evident for a key secondary endpoint - the average change from the baseline in the ALS functional rating scale (ALSFRS-R) - within a pre-specified subgroup of participants with a baseline score of at least 35. This crucial change was attributed to a correction in the efficacy model utilized in the original analysis. When the pre-specified model was employed in the revised analysis, marked improvements surfaced in all patient subgroups with ALSFRS-R baseline scores between 26 to 35 after NurOwn treatment.
The reevaluated data provides promising signals. The significant improvement in a key secondary endpoint, alongside evidence of beneficial effects on diverse patient subgroups, hints at the potential effectiveness of NurOwn. However, the lack of statistically significant results in the wider Phase 3 trial population might present hurdles in the approval process. Additionally, the reliance on subgroup analyses when assessing significant clinical improvements could potentially stem from sophisticated numerical crunching, rather than being indicative of an authentic, reproducible trend.
The chances of NurOwn receiving approval remain uncertain. The promising subgroup data and solid safety profile might be enough to gain approval under specific circumstances, yet the absence of statistically significant results in primary endpoints in the larger population presents a formidable challenge. Furthermore, the approval process depends significantly on the company's dialogue with the FDA. In 2021, the FDA did not see the data as sufficient evidence to back a BLA but did not discourage a future submission either. This implies that while approval is not assured, there may be scope for negotiation and potential submission of further data to bolster the application. Nonetheless, the final verdict lies in the hands of the regulatory authorities, who navigate the complex and often unpredictable world of drug approval.
Brainstorm Pursues FDA Approval through 'File Over Protest' Protocol
"File Over Protest" is a process within the U.S. Food and Drug Administration's (FDA) drug approval protocols that Brainstorm has chosen to follow for the review of their Biologics License Application (BLA) for NurOwn. This procedure allows a pharmaceutical company to insist that the FDA reviews an application that was initially refused, as was the case with Brainstorm's BLA.
The FDA initially refused to file Brainstorm's application as it believed the application lacked the necessary data to establish NurOwn's safety and efficacy. However, through the "File Over Protest" process, Brainstorm maintains that the existing data is sufficient and demands that the FDA review it.
Choosing to "File Over Protest" is not a decision Brainstorm would have taken lightly. Such a choice could potentially create tension between Brainstorm and the FDA and could lead to a more stringent review process. Furthermore, even though Brainstorm has chosen to "File Over Protest," this does not guarantee approval for NurOwn. The treatment still needs to meet the FDA's rigorous standards for safety and efficacy.
My Analysis & Recommendation
Investors in Brainstorm should prepare for a significant amount of uncertainty. The fate of NurOwn is at a critical juncture with the ADCOM meeting on September 27, 2023, looming in the near future. The FDA's review of the new and corrected data from Brainstorm's Phase 3 trials will be instrumental in defining the future of the drug. Any indications regarding the FDA's position towards the company's addressed CMC issues and corrected trial efficacy data will be crucial.
While there is always a degree of uncertainty surrounding drug approvals, Brainstorm's situation is particularly precarious. I believe the company's decision to 'File over Protest' can be seen as a desperate 'Hail Mary' attempt to push NurOwn over the regulatory finish line. This move could lead to a more stringent and critical review process, which could potentially complicate Brainstorm's interactions with the FDA in the future. Moreover, the lack of statistically significant results in the broader patient population casts a shadow over the promising results seen in specific subgroups.
Brainstorm's financial position further exacerbates these concerns. With a cash balance of approximately $2.2 million as of March 31, 2023, and quarterly expenditures exceeding this amount, the company may need to secure additional capital in the immediate future. Whether through debt, issuing more equity, or forging partnerships, these actions could dilute the value of existing shares and stress the company's financial health.
While the potential breakthrough that NurOwn represents in the treatment of neurodegenerative diseases cannot be denied, the combination of regulatory and financial hurdles paints a risky investment landscape. The optimism stemming from the corrected trial data and the potential of the company's unique cell therapy platform needs to be tempered by the harsh realities of the approval process and the company's precarious financial position.
Given these uncertainties, the current recommendation for Brainstorm is a "Sell". The need for additional capital, coupled with the 'Hail Mary' nature of their 'File over Protest' action and uncertain approval odds, creates a high-risk scenario for investors. Therefore, despite the potentially transformative nature of NurOwn, investors might want to exit at this stage until there is more clarity on the FDA's stance and the company's financial outlook.
Risks to Thesis
When the facts change, I change my mind.
There are several potential risks involved that could significantly impact my ‘Sell’ recommendation.
Regulatory Approval: A primary risk is the potential for unexpected positive regulatory outcomes. If the FDA accepts the resubmitted BLA and ultimately approves NurOwn, the company's stock value could significantly rise.
Subgroup Efficacy: The company has noted a significant treatment difference in specific subgroups of ALS patients, as per the corrected trial data. If future data continues to support these results, it could increase the chances of approval, which might boost the company's valuation.
Successful Financing: Currently, Brainstorm's cash position is a concern. However, if the company manages to secure substantial funding without significant dilution of shares, it could enhance the financial outlook and strengthen the company's position, potentially leading to a rise in the stock price.
Strategic Partnerships: Brainstorm may also form strategic partnerships or collaborations that could provide additional financial support, resources, and credibility, potentially increasing the company's valuation.
Promising Future Pipeline: While the current focus is on NurOwn, Brainstorm might have other promising candidates in their pipeline. Future successes in the development of these candidates could increase the company's worth.
Market Sentiment: Investor sentiment can often defy fundamental analysis. If investor sentiment becomes overwhelmingly positive, the stock price could rise, regardless of the current uncertainties.
This article was written by
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
This article is intended to provide informational content only and should not be construed as personalized investment advice with regard to "Buy/Sell/Hold/Short/Long" recommendations. Any predictions made in this article regarding clinical, regulatory, and market outcomes are the author's opinions and are based on probabilities, not certainties. While the information provided aims to be factual, errors may occur, and readers should verify the information for themselves. Investing in biotech is highly volatile, risky, and speculative, so readers should conduct their own research and consider their financial situation before making any investment decisions. The author cannot be held responsible for any financial losses resulting from reliance on the information presented in this article.
Seeking Alpha's Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.
