Cogent Biosciences: APEX Part 2 Launched, ErbB2 And FGFR2 On Track
Summary
- Cogent Biosciences recently presented updated preclinical findings on the ErbB2 and FGFR2 programs, showcasing a commitment to developing novel therapies for uncharted disease areas.
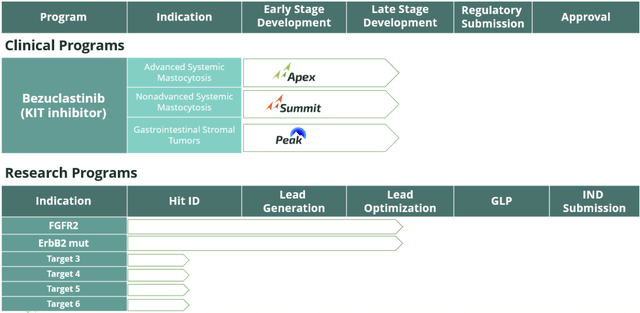
- The company launched Part 2 of the APEX trial to evaluate the effectiveness of bezuclastinib in Advanced Systemic Mastocytosis, with ongoing enrollment expected to finish by the end of 2024.
- Despite short-term losses, Cogent's strategic investments in R&D will ultimately yield long-term benefits.
byakkaya
Cogent Biosciences (NASDAQ:COGT) offers a compelling investment opportunity for those seeking exposure to a pioneering biotech company revolutionizing the development of precise therapies for genetically influenced diseases. By focusing on targeted treatments such as bezuclastinib and investigating novel ErbB2 and FGFR2 inhibitors, Cogent Biosciences showcases enormous potential in addressing significant unmet medical needs in the market.
Recent years have seen the growth of precision medicine: an innovative approach that utilizes genetic and molecular insights to effectively treat rare and difficult-to-treat diseases. Cogent Biosciences, Inc. is a leading biotech company with a commitment to transforming treatment for patients affected by genetically driven disorders. Its financial position is strong, and it has dedicated resources towards research and development (R&D); this focus on unexplored therapeutics makes it an interesting long-term investment prospect in today's booming biopharmaceutical market.
In this article, we'll evaluate business elements of Cogent Biosciences that make it attractive to investors. Namely, its healthy balance sheet, growing research and development budget, performance in clinical studies, and pioneering drug bezuclastinib-compared against other industry players like Blueprint Medicines (BPMC) and Novartis AG (NVS). Through this analysis, we hope to provide investors with insight into why Cogent Biosciences may be well worth investing in, due to its unique combination of benefits and potential rewards.
Financial State
The financial position of Cogent Biosciences, Inc. displays a solid prospect for investors interested in a pioneering organization committed to creating transformative therapies. In Q1 2023, the firm's cash and cash equivalents amounted to an astounding $220.3 million, showcasing financial durability despite a $39 million expense. Furthermore, the current cash reserves are anticipated to support operations and capital needs until 2025 at least. The fiscal stability of the company highlights a well-considered and composed strategy for growth-an encouraging signal for discerning investors.
Cogent Biosciences is committed to growth, as evidenced by their $36.0 million R&D expenditure in Q1 2023-a significant increase from the $25.5 million in the same quarter of 2022. Clinical trials such as APEX, SUMMIT and PEAK have driven this boost in spending, giving investors faith that the company can bring cutting-edge treatments to those in need. Substantial non-cash stock compensation expenses within R&D also rose, moving up from $1.9 million in 2022's first quarter to $3.0 million in 2023, with the expansion confirming the firm's determination to reward and incentivize its employees for continually excelling.
General and administrative (G&A) costs saw likewise progressive development, rising from $5.9 million in 2022's initial quarter to $7.2 million during the present time-frame. Even if potential investors are daunted by this rise, it actually reveals the business is getting larger, which involves escalated operational expenditures. Happily, the financial investment in non-cash stock compensation expenses within G&A moved upward as well, improving from $2.3 million in 2022 to $2.9 million in Q1 2023, demonstrating Cogent Biosciences' dedication in utilizing top talent.
The reported net loss of $38.6 million in Q1 2023, compared to $30.6 million during the same period last year, must not discourage investors. This loss occurs primarily due to investments made into R&D and other areas of business. As the organization continues exploring scientific advancement, these temporary losses should provide rewarding returns for shareholders in coming years.
Cogent's Solid Pipeline
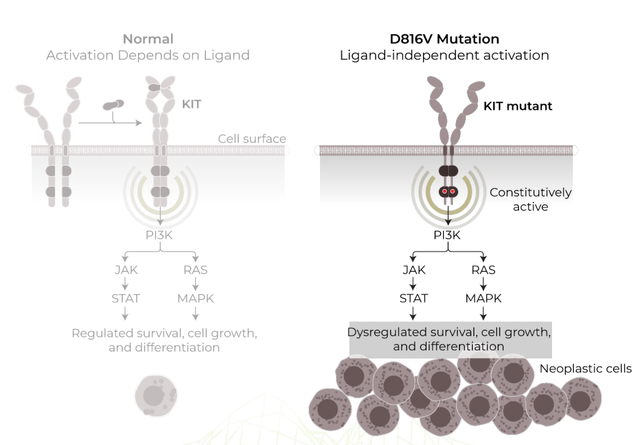
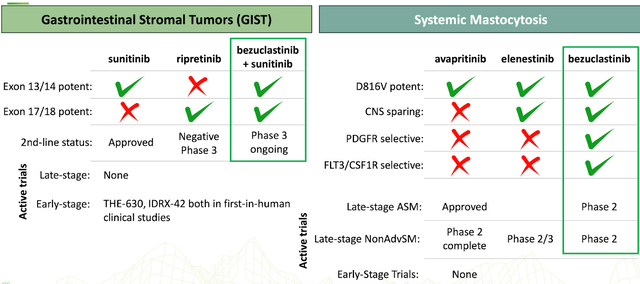
Cogent Biosciences, Inc. is committed to creating precise treatments for genetically influenced illnesses. Their primary clinical program is centered on bezuclastinib, a selective tyrosine kinase inhibitor that specifically targets KIT D816V mutations and other KIT exon 17 mutations. These mutations play a crucial role in driving systemic mastocytosis and advanced gastrointestinal stromal tumors (GIST).
In 2023, the company launched multiple important clinical studies and initiatives, including the commencement of part 2 of the Phase 2 APEX trial. This trial aims to assess bezuclastinib's effectiveness in treating advanced systemic mastocytosis (AdvSM). Part 2 is projected to enroll approximately 65 patients and gather data to support regulatory submissions, with the enrollment process expected to conclude by the end of 2024. Furthermore, Cogent Biosciences showcased preclinical data featuring insights into their ErbB2 and FGFR2 research programs.
The firm attained approvals from European regulatory bodies to initiate the Phase 2 SUMMIT trial for patients with non-AdvSM, leading to the establishment of clinical trial locations throughout the European Union. The FDA also granted Orphan Drug Designation to bezuclastinib for treating various forms of mastocytosis, including systemic mastocytosis.
Looking forward, the company plans to unveil initial clinical findings from the SUMMIT trial and Part 1 of the APEX trial during the latter half of 2023 at a scientific conference. Besides bezuclastinib, the research team at Cogent Biosciences is actively working on a portfolio of innovative targeted treatments focusing on FGFR2 and ErbB2. These therapies aim to provide effective alternatives for patients suffering from severe genetically driven diseases.
Promising Preclinical Findings from AACR
Cogent Biosciences recently presented updated preclinical findings from its pipeline projects, which include a unique EGFR-sparing, brain-penetrant ErbB2 inhibitor and a specialized FGFR2 program. Additionally, Cogent launched Part 2 of the APEX trial for their drug bezuclastinib, intended to treat AdvSM.
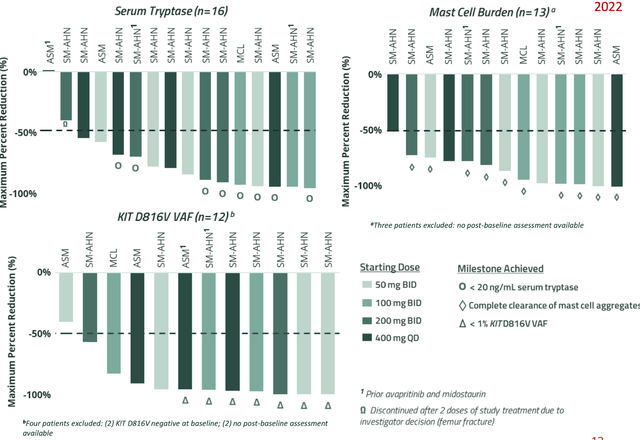
The organization's scientists are dedicated to identifying and progressing potential top-tier novel therapies for rare disease populations with significant unmet medical requirements. Due to bezuclastinib's impressive clinical performance, safety, and tolerability, Part 2 of the APEX trial in AdvSM began with a once-daily 150 mg dosage. Updates from both the APEX and SUMMIT trials are expected in the latter half of 2023, and updated findings from the PEAK GIST patient trial are anticipated within the quarter.
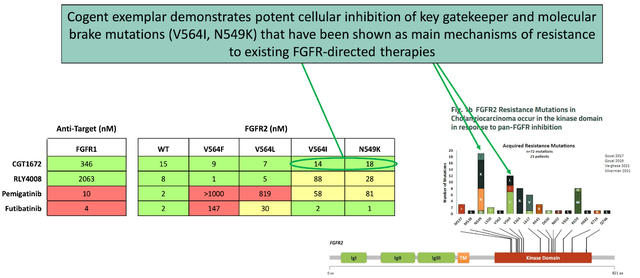
The AACR poster presentations revealed several key advancements. One presentation detailed a selection of innovative compounds that powerfully inhibit crucial ErbB2 mutations while avoiding EGFR inhibition. These compounds display notable benefits compared to the currently approved benchmark compound, tucatinib.
Another presentation disclosed the first documented evidence of a reversible, selective FGFR2 inhibitor that covers activating and emerging resistance mutations and spares FGFR1 inhibition. This preclinical information demonstrates equal coverage across essential gatekeeper and molecular brake mutations in FGFR2 without any signs of FGFR1-related hyperphosphatemia at effective plasma concentrations.
As for the APEX trial, Part 2 of the study will now incorporate supplementary patient groups to display the range of AdvSM patients who may benefit from bezuclastinib.
These developments emphasize Cogent Biosciences' dedication to the development of state-of-the-art therapies centered on genetically driven diseases. The progression in both the ErbB2 and FGFR2 programs, as well as the advances in the APEX trial, exemplify the company's commitment to delivering innovative treatment alternatives for patients with rare disorders and unmet medical demands.
Possibility of Risks
Bezuclastinib displays potential in targeting genetically driven diseases such as systemic mastocytosis and gastrointestinal stromal tumors. However, it is critical to acknowledge the possibility of resistance mechanisms emerging during treatment. Additional KIT mutations or alternative signaling pathways may arise, rendering bezuclastinib less effective over time. This highlights the need for continuous monitoring and adaptation in the treatment strategy to overcome potential resistance mechanisms.
It is also essential to consider the complex molecular pathways involved in these diseases. Mastocytosis, driven by the uncontrolled proliferation of mast cells due to the KIT D816V mutation, and GIST, which relies on oncogenic KIT signaling, may involve varied mutations and alterations that can impact treatment response. Therefore, understanding the diverse genetic and molecular factors that contribute to disease progression and treatment resistance is crucial.
In the context of Cogent Biosciences' ErbB2 and FGFR2 research programs, potential challenges related to drug specificity and selectivity must be considered. It is vital to achieve high specificity in targeting the intended receptor or mutation while minimizing off-target effects. This helps to avoid unintended interactions with other crucial signaling pathways, reducing the risk of toxicity concerns.
Another potential concern with targeted therapies is the possibility of adverse effects on normal physiological functions. In the case of systemic mastocytosis, mast cells play important roles in immune responses and regulation of various physiological processes. Therefore, balancing the inhibition of aberrant mast cell proliferation and preservation of normal mast cell function is essential to prevent detrimental effects on the immune system and other biological processes.
Lastly, challenges associated with clinical trials and drug development must be addressed. As the company progresses through different phases of clinical trials, it is crucial to consider factors such as patient recruitment, trial design, and potential complications that could affect the timely completion and success of the trials. Furthermore, the safety profile of the drugs and any adverse events should be closely monitored throughout the trials.
Cogent Outshines Competitors
One of the competitors in the space of precision therapies for genetically driven diseases is Blueprint Medicines, which focuses on targeted therapies for genomically defined diseases. Their product avapritinib, a KIT and PDGFRA inhibitor marketed as Ayvakit, received FDA approval for treating adults with GIST harboring a PDGFRA exon 18 mutation. Although avapritinib seems to be effective in patients with GIST, it lacks the specificity of bezuclastinib towards the KIT D816V mutation, which is crucial for systemic mastocytosis treatment. In contrast, Cogent Biosciences' bezuclastinib being a selective tyrosine kinase inhibitor provides a superior therapeutic option for patients suffering from systemic mastocytosis, emphasizing its potential to be a more targeted and precise therapy.
Another competitor is Novartis, which developed midostaurin, a multitargeted kinase inhibitor marketed as Rydapt. Midostaurin was approved by the FDA for aggressive systemic mastocytosis (ASM), systemic mastocytosis with associated hematological neoplasm (SM-AHN), or mast cell leukemia (MCL) with mild to moderate symptoms. However, midostaurin inhibits a broad range of kinases and is not as selective in targeting the KIT D816V mutation as bezuclastinib. In addition, non-specific kinase inhibition could lead to potential off-target effects, resulting in a reduced safety profile. In contrast, bezuclastinib's selectivity enhances its potential to provide better efficacy and tolerability, making it a superior choice for patients dealing with systemic mastocytosis and advanced GIST.
Cogent Biosciences also seems to be ahead of its competitors in the development of targeted therapies for ErbB2 and FGFR2-driven diseases. For example, the EGFR-sparing, brain-penetrant ErbB2 inhibitor being developed by Cogent Biosciences has demonstrated higher specificity than the current approved drug, tucatinib, which is marketed by Seagen (SGEN) for HER2-positive breast cancer patients. Additionally, Cogent's selective reversible FGFR2 inhibitor presents a novel approach in addressing unmet medical needs in the FGFR2-driven disease area, offering advantageous properties like lower risk of hyperphosphatemia compared to non-selective inhibitors.
Conclusion
Cogent Biosciences yields immense potential for investors searching to capitalize on breakthrough precision therapies addressing genetically influenced diseases. The company's unwavering commitment to transforming patient care through state-of-the-art research and development substantiates their position as a front-runner in biotechnology innovation. As a testament to their forward-looking financial strategy, Cogent Biosciences' remarkable cash reserves prove their capability to weather potential storms while continuously investing in core operations and R&D endeavors.
Our bullish outlook on Cogent Biosciences is further buoyed by the firm's strategic growth in R&D expenses to support competitive clinical trials like APEX, SUMMIT, and PEAK-all crucial to fulfilling their underlying mission of delivering innovative treatments for rare, uncharted diseases. The expansion of non-cash stock compensation in both the R&D and general and administrative segments illustrates a strong employee-focused culture that positions the company to attract top talent, ensuring longevity in their race to make transformative therapies available to those in need.
As for the company's approach in precision medicine-targeting therapies like bezuclastinib, a game-changing drug for systemic mastocytosis, and advanced GIST patients-reveals a competitive advantage over industry peers, such as Blueprint Medicines and Novartis. The commitment to further investigate novel therapies for genetically driven diseases, such as their ErbB2 and FGFR2 research programs, displays the company's drive to make a substantial impact in areas with significant unmet medical needs.
While short-term losses may deter some investors, we are optimistic that Cogent Biosciences' strategic investments in R&D, growth, and employee retention will ultimately yield lucrative long-term benefits and substantial market positioning as they break new ground in precision medicine. In our opinion, Cogent Biosciences' unique combination of financial stability, clinical-trial advancement, and cutting-edge innovation fosters ample opportunities for rewarding investments in the world of biotech. Investing in Cogent Biosciences is not simply investing in a biotech company, but rather, a vision: to transform treatment methodologies and redefine the realm of precision medicine.
This article was written by
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Seeking Alpha's Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.