Coya Therapeutics: Developing Treatments For Serious Neurodegenerative And Autoimmune Diseases

Summary
- Coya Therapeutics’ pipeline candidates focus on improving the quantity and functionality of regulatory T cells to alleviate the inflammatory processes associated with the progression of neurodegenerative and autoimmune diseases.
- Regulatory T cells, or Tregs, constitute a significant class of T-cells that utilize their anti-inflammatory properties to maintain immune balance or homeostasis.
- The release of the data in patients with AD follows the recent results from the COYA 302 Proof-of-concept study in patients with ALS that showed amelioration of disease progression.
- The expected release of results from the COYA 301 Proof-of-concept study in patients with AD in May 2023 serves as a substantial near-term catalyst with the potential to elevate the company's market value.
- Coya’s pipeline candidates have undergone the initial human trials depicting the efficacy and safety of the company’s Treg-modulating therapies in slowing the progression of ALS and Alzheimer’s disease (AD).
Artur Plawgo
Introduction and Investment Thesis
My interest in Coya Therapeutics (NASDAQ:COYA) stems from its innovative and straightforward approach to modulating the function of regulatory T cells (Tregs) to address the underlying inflammatory processes in complex neurodegenerative and autoimmune conditions with significant unmet medical needs. The company's diverse pipeline of Treg-modulating candidates seeks to restore immune balance by targeting inflammation, a common and critical factor in the progression of numerous neurodegenerative diseases (NDDs) and autoimmune diseases. Coya aims to treat 1.7 million patients across the U.S., with a total addressable market exceeding $7 billion. Moreover, Coya recently reported encouraging results from its proof-of-concept study in ALS patients. COYA 302 effectively slowed disease progression, as demonstrated by a lack of decline at 24 weeks and minimal decline at 48 weeks after initiation of treatment in Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) scores compared to score values at baseline. A higher ALSFRS-R score represents better functional status in ALS patients. The positive clinical results were consistent with the increased Treg function observed over the treatment period.
Given this context, this article is a detailed explanation of Coya Therapeutics' approach to developing a treatment for various fatal chronic conditions. The article is divided into three parts.
The first is an overview of the company, and three further topics deal with understanding Tregs and their functionality.
Further topics deal with understanding the company’s pipeline candidates.
The final few topics are aimed at explaining the market for ALS and AD, the company’s financial position, concluding statements, and sensitivities.
About the Company
Coya Therapeutics is a biotechnology company concentrating on the development of innovative pharmaceutical solutions that modulate regulatory T cells (Tregs) to address severe neurodegenerative, autoimmune, and metabolic disorders. Tregs play a pivotal role in preserving immune system equilibrium and averting detrimental responses. Established in 2020, the company has since devised numerous strategies to enhance the functionality of Tregs. Coya Therapeutics' primary therapeutic modalities encompass 1) Treg-augmenting biological agents, 2) Treg-derived exosome technology, and 3) autologous Treg cell therapy. These modalities are categorized into the "300 Series," "200 Series," and "100 Series" product candidates, respectively.
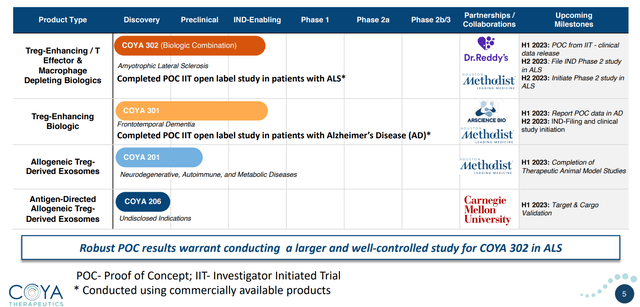
Product Pipeline (Company Presentation)
Exhibit 1: Product Pipeline Source: Company Presentation
The company has made significant strides in its diverse pipeline of candidates, showcasing various therapeutic approaches, all centered on capitalizing on the role of regulatory T Cells in fighting inflammation and restoring immune balance. By concentrating on an array of therapeutic methodologies and targeting afflictions with substantial unmet medical demands, Coya Therapeutics aspires to mitigate risks and bolster the likelihood of securing regulatory approval and ultimately commercializing its products.
Orchestrating Immune Equilibrium: The Influence of Tregs in Preserving Immune System Stability
Before getting into the intricacies of regulatory T cells, simply called Tregs, it is essential to comprehend the basics of T cells, their crucial functions, and their various subtypes. T lymphocytes are a type of white blood cell responsible for detecting and eliminating harmful pathogens and coordinating immune responses. They are classified into different types based on their unique roles and the presence of different surface protein expressions, which are CD4 T cells (consisting of helper cells and regulatory cells), and CD8 T cells (also known as cytotoxic T cells), among others.
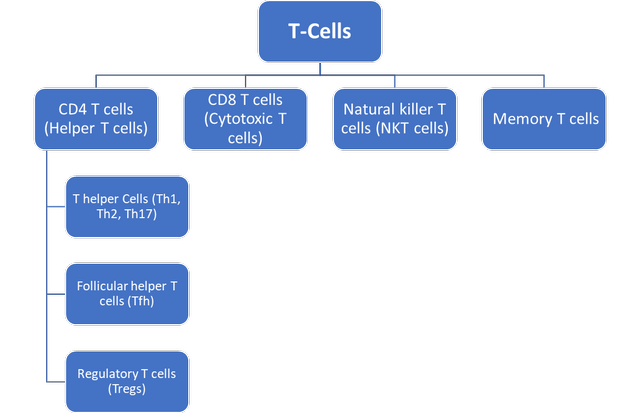
Subtypes of T-Cells (own work)
Exhibit 2: Subtypes of T-Cells. Source: (Own Work)
Effector T cells include helper and cytotoxic T cells, which actively participate in immune responses against infections and diseases by directly attacking infected cells, producing cytokines, or assisting other immune cells. For example, helper T cells stimulate the activity of B cells to produce antibodies, stimulate macrophages to fend off foreign pathogens, and play an important role in activating cytotoxic T cells. On the other hand, cytotoxic T-cells recognize and kill infected and/or mutated cells, inducing cell apoptosis.
Regulatory T cells form a subpopulation of CD4+ T cells that plays a crucial role in upholding immunological balance and preventing autoimmunity. They are differentiated by the presence of the transcription factor forkhead box P3 (FOXP3), a vital molecular factor that directs their development, function, and stability. Tregs regulate the activity of other immune cells, such as effector T cells, to prevent the immune system from accidentally attacking the body’s own tissue. This process of preventing the development of autoimmune conditions is called immune self-tolerance. Tregs achieve this by releasing immunosuppressive cytokines like IL-10 and TGF-β, and dampening the immune response by direct contact. These mechanisms collectively mitigate the activation, proliferation, and effector responses of immune cells, thereby restraining excessive or aberrant immune reactions. Tregs also play a key role in resolving inflammation and helping tissue repair by controlling local cytokines and managing the actions of other immune cells in the surrounding tissue. This is especially important in areas where excessive immune responses could cause serious damage.
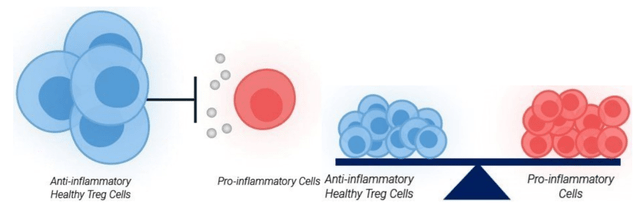
Tregs Ensuring Immune Equilibrium (company presentation)
Exhibit 3: Tregs Ensuring Immune Equilibrium. Source: Company Presentation
Both effector T cells, which are pro-inflammatory, and Tregs, which are anti-inflammatory, are vital components of the immune system, working together to establish homeostasis or immune equilibrium. This delicate balance allows for an effective immune response against foreign pathogens while preventing collateral damage to the body's own tissues. By maintaining harmony between these two types of T cells, the immune system can adapt and respond to various challenges, ensuring overall health and protection against potential threats. Additionally, this natural equilibrium contributes to efficient tissue repair and recovery after an injury, fostering resilience in the face of infections and diseases.
The Impact of Dysfunctional Tregs on Inflammation and the Development of Chronic and Progressive Disorders
The above discussion highlights the role of Tregs in maintaining immune homeostasis. However, when Tregs become dysfunctional, it can create an immune imbalance, giving rise to a number of life-threatening diseases.
Tregs become dysfunctional when they have lost their capability to regulate immune responses effectively, resulting in an imbalanced immune system and an increased risk of developing autoimmune and neurodegenerative diseases characterized by increased inflammation. The reasons behind dysfunctional Tregs remain multifaceted and might involve a combination of environmental, health-related, and genetic factors. In addition to these aberrations, mutation in the FOXP3 gene may also contribute to Treg dysfunction.
Consequences
The cellular dysfunction of Tregs has detrimental consequences, as the immune system is unable to differentiate between harmful and healthy cells. This leads to a situation of chronic inflammation, wherein the pro-inflammatory cytokines/ proteins (IL-6, IL-1, and TNF-α) also contribute to the death of healthy cells.
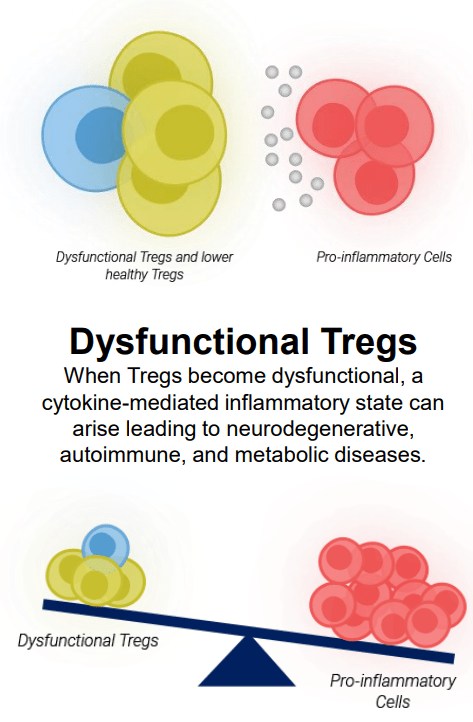
Immune Disequilibrium Due to Dysfunctional Tregs (company presentation )
Exhibit 4: Immune Disequilibrium Due to Dysfunctional Tregs. Source: Company Presentation
Abnormal and chronic inflammatory responses are a common link in the progression of various serious neurodegenerative and autoimmune disorders. Dysfunctional Tregs are unable to effectively suppress the immune response initiated by pro-inflammatory cells like cytotoxic T cells. This leads to a persistent state of inflammatory response, causing tissue damage and a heightened risk of developing comorbidities. Comprehending the role of Tregs in the development and progression of progressive and often fatal conditions is important in developing effective and safe treatment modalities. In my previous article on BioVie Inc., I highlighted the growing interest in neuroinflammation and insulin resistance as a potential regulatory pathway of disease pathogenesis and increased interest in understanding the complex interplay between neuroinflammation and neurodegeneration.
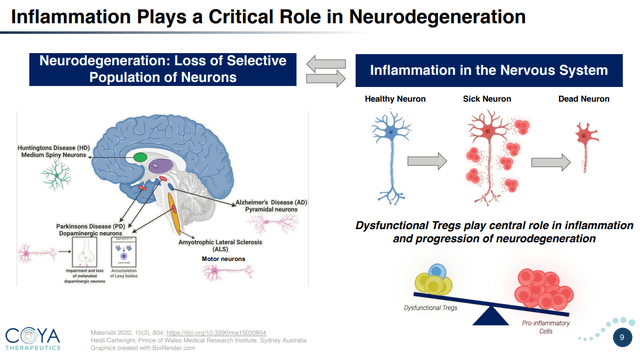
Neuroinflammation and Neurodegeneration (Company presentation)
Exhibit 5: Neuroinflammation and Neurodegeneration. Source: Company Presentation
The Interplay of Neuroinflammation, Dysfunctional Tregs, and Disease Progression in Alzheimer's and Other Progressive Debilitating Conditions
The role of neuroinflammation in the progression of neurodegenerative diseases, including Alzheimer’s, has been widely discussed in numerous research papers. Specifically, Alzheimer's starts with the buildup of amyloid-β plaques and neurofibrillary tangles composed of misfolded tau proteins, leading to the eventual decline and demise of neurons. Neuroinflammation plays a critical role in this context, as the activation of microglial cells is a key factor in Alzheimer's-related inflammation. As the brain's innate immune cells, microglia continuously observe their environment and respond to changes. In Alzheimer's disease, the development of amyloid-β plaques sets off microglial activation, which initially assists in reducing and clearing these deposits. However, persistent activation of microglia can cause persistent neuroinflammation, worsening Alzheimer's over time.
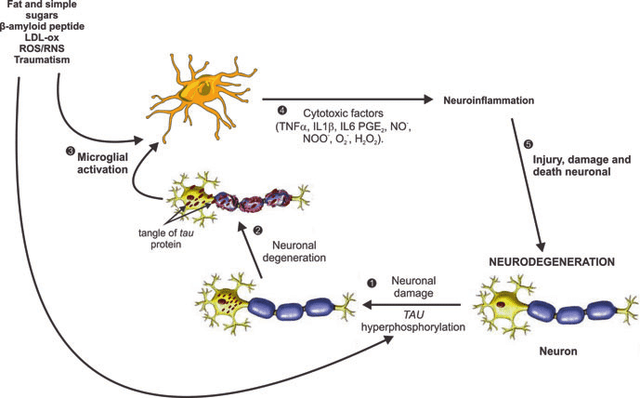
Microglia Activation and Neuroinflammation Pathway in AD (Matinez L. et al)
Exhibit 6:Microglia Activation and Neuroinflammation Pathway in AD. Source: Martínez L. et al.
In a healthy brain, a balance exists between pro-inflammatory (M1 microglia) and anti-inflammatory (M2 microglia) cells, maintaining homeostasis and controlling inflammatory processes. Regulatory T cells (Tregs) also play a vital role in preserving immune balance by suppressing excessive inflammation and fostering immune tolerance. Toxic factors like amyloid β plaques can cause microglia to adopt a detrimental M1 pro-inflammatory state, which releases harmful substances that damage neurons and accelerate disease progression. The chronic activation of M1 microglia can lead to ongoing inflammatory responses or neuroinflammation. Through their anti-inflammatory actions, regulatory T cells can decrease inflammation caused by activated microglia. However, in Alzheimer's disease, dysfunctional Tregs may further contribute to the disruption of immune balance and continuing inflammation.
In the context of neurodegenerative, autoimmune, and metabolic disorders, dysfunctional Tregs have been shown to exacerbate disease progression through various mechanisms. For example, in Alzheimer's disease, a study demonstrated that a Treg deficiency led to an increase in pro-inflammatory cytokines and accelerated cognitive decline in mouse models. However, increasing regulatory T cells with low-dose IL-2 treatment enhanced microglial response to amyloid-β plaques and restored cognitive function. The findings suggest the potential of repurposing IL-2 as a novel immunotherapy targeting regulatory T cells to treat Alzheimer's disease.
Enhancing Treg function has also shown promise in alleviating disease symptoms in experimental models of multiple sclerosis and Type 1 diabetes. Furthermore, in metabolic disorders like obesity and Type 2 diabetes, dysfunctional Tregs contribute to chronic inflammation, insulin resistance, and disruption of metabolic homeostasis, which exacerbate these conditions. The pursuit of more engaging insights supported by factual data and examples will be crucial in unlocking the full potential of Treg-based therapies for various disorders.
Therapeutic Modality for Autologous Regulatory T Cells (Tregs) (Coya Therapeutics’ 100 Series): Clinical Proof of Safety and Efficacy
COYA 101 is the company’s autologous Treg cell therapy that has been evaluated in patients with amyotrophic lateral sclerosis (ALS). The COYA 101 clinical trials have provided preliminary evidence supporting the case for regulatory T cells as potent therapeutics for neurodegenerative diseases, supporting the company’s diversified pipeline of 200 and 300 series of Treg-modulating candidates.
Clinical Trial Results
The Phase 1 (n=3) and Phase 2a (n=8) clinical trials for COYA 101 demonstrated the potential of Treg-based therapy in the treatment of ALS. The trials primarily focused on evaluating the biological activity, safety, tolerability, and preliminary clinical efficacy of COYA 101. In the Phase 1 trial, patients showed improvements in certain biological markers, such as pro-inflammatory cytokines and oxidative stress biomarkers, suggesting the therapy might have a positive impact on neuroinflammation, resulting in amelioration of disease progression. The Phase 2a results indicated an adequate tolerability profile and potential efficacy, as 75% (n=6 of n=8) of the patients experienced halted or slowed disease progression during the 24-week open-label phase of the Phase 2a trial. Both trials demonstrated a favorable safety and tolerability profile with no serious adverse events (AEs).
Moreover, the evaluation of pro-inflammatory and oxidative stress serum biomarkers suggested that certain biomarker levels correlate with disease progression and severity, potentially helping to identify patients who may benefit more from COYA 101 treatment.
Development Status
The COYA 101 clinical trial results demonstrated the potential for Treg-based therapies and that targeting Tregs has a meaningful effect on ALS progression. Based on the lessons learned from the Treg cell therapy studies, Coya Therapeutics has prioritized the development of the biologic programs (300 series) that could address Treg dysfunction in a more scalable, cost-effective manner, as an off-the-shelf product with convenient subcutaneous administration. Furthermore, the company aims to advance COYA 101 into a Phase 2b trial through non-dilutive funding sources or other strategic options allowing continued development without compromising the progress of its other Treg candidates.
The positive efficacy and safety data exhibited by COYA 101 in Phase 1 and Phase 2a ALS clinical trials, although limited, are encouraging. However, given the constraint of resources at their disposal, Coya’s management believes the optimal usage of shareholder’s capital would be to move forward with the biologic programs (COYA 300 series). This likely indicates that management has strong confidence in the 300 and 200 series candidates and believes they might have a higher possibility of success. Additionally, the company’s other multi-modality approaches to leveraging Tregs’ anti-inflammatory mechanism of action (MoA) are much more cost-effective and hold the potential of broader market applicability.
COYA 301 and COYA 302: An Innovative and Novel Strategy for Addressing Neurodegenerative Disorders
Until now, the discussion has highlighted Tregs’ anti-inflammatory function and ability to hinder the neuroinflammation pathways involved in the progression and severity of various neurodegenerative diseases. These findings paved the way for Coya Therapeutics to advance its scalable and cost-effective 300 series of Treg-augmenting biological investigational agents. With these product candidates, the company aspires to create biologic therapies that minimize inflammation and promote self-tolerance in select neurodegenerative and autoimmune diseases by boosting the suppressive and immunomodulatory capabilities of Treg cells.
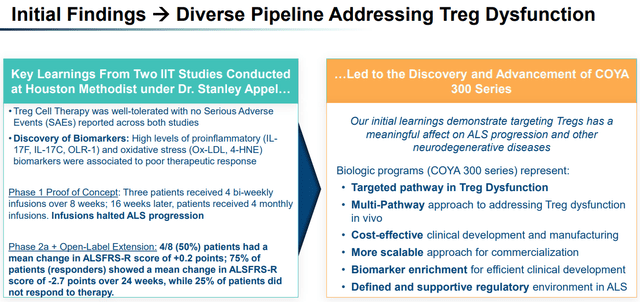
Initial Findings Aiding the Progress of Diverse Pipeline (Company presentation)
Exhibit 7:Initial Findings Aiding the Progress of Diverse Pipeline. Source: Company Presentation
Both COYA 301 and 302 leverage low-dose IL-2 treatment in an effort to enhance the population and function of regulatory T cells. Interleukin-2 (IL-2) is an important cytokine/ protein responsible for regulating the immune response and immune tolerance by promoting the activation, growth, and proliferation of T cells. Since its discovery in the 1970s, our understanding of IL-2 has evolved dramatically. Initially thought to be a pro-inflammatory factor stimulating effector cells to fight pathogens and tumors, it was FDA-approved as a high-dose cancer treatment in the 1990s. However, surprising discoveries revealed its vital role in immune tolerance and its ability to stimulate the growth and function of regulatory T cells. With its two-faceted function and substantial improvement in technology, multiple biotechnology companies are racing toward developing an engineered version of IL-2 either as an anti-cancer treatment (by stimulating effector cells) or as a treatment for autoimmune disease (by stimulating Tregs). Low-dose IL-2 therapy has been extensively researched for the treatment of various neurodegenerative diseases (NDDs), autoimmune diseases, and alloimmune diseases. The treatment exhibits a multi-modal mechanism of action (MoA) that involves:
Stimulating the expansion and activation of Tregs
Inhibiting the activation of effector IL-17-producing helper T (TH17) and follicular helper T (TFH) cells and other key pro-inflammatory participants, which are key players in increasing and maintaining neuroinflammation.
Activating and driving proliferation and memory formation of CD8+ T cells, which are essential for eliminating infected cells and controlling intracellular infections.
Low-dose IL-2 therapy has been assessed in numerous clinical and pre-clinical studies involving diverse NDDs and autoimmune diseases. Here is a synopsis of the outcomes from a selection of these clinical and pre-clinical investigations.
Alzheimer’s disease: The study found that IL-2 levels were decreased in hippocampal biopsies of Alzheimer's patients and that treating APP/PS1E9 transgenic mice with IL-2 resulted in improved synaptic plasticity, increased spine density, decreased amyloid plaque load, and recovery of memory deficits. These results suggest that IL-2 could help alleviate the typical characteristics and symptoms of Alzheimer's disease.
Type 1 diabetes: In a study conducted on non-obese diabetic (NOD) mice with established Type 1 diabetes, the administration of recombinant human IL-2 for five consecutive days at a dose of 25,000 IU resulted in disease remission. The treatment improved the function of pancreatic regulatory T cells, suppressing the activity of local T cells producing type II interferon (IFN-γ) and halting the progression of the mice’s diabetes.
ALS: The study investigated the effects of low-dose IL-2 on gene expression in the peripheral blood of patients with amyotrophic lateral sclerosis. The results showed that IL-2 promoted Treg expansion and suppressed immune activation. Patient-derived astrocytes treated with IL-2 exhibited potentially protective changes, including a reduction in oxidative stress, which suggested a reduction in ALS astrocyte-mediated motor neuron toxicity. The study also identified predictive biomarkers that could help determine patient responsiveness to IL-2.
About COYA 301 and 302
COYA 301, a biologic therapy and low-dose interleukin 2 (IL-2) product candidate, has been developed to be administered subcutaneously (beneath the skin) with the aim of enhancing regulatory T cell functionality and increasing their numbers within the body. This treatment is targeted at frontotemporal dementia, an orphan neurodegenerative disease with high unmet needs in which Treg function is impaired. By raising the number and function of functional Tregs, the company aims to activate and boost the anti-inflammatory mechanism, thus hindering the neuroinflammatory pathways of disease progression. Additionally, the subcutaneous delivery method of COYA 301 enables patients to administer the treatment in the comfort of their own homes, which offers both convenience and cost-effective advantages compared to current treatments that necessitate hospital administration.

COYA 301 MoA (Company presentation)
Exhibit 8:COYA 301 MoA. Source: Company Presentation
On the other hand, COYA 302 is composed of two components: COYA 301 and Abatacept (CTLA4-Ig), a fusion protein that downregulates pro-inflammatory cells. With a dual mechanism of action, the biologic aims to increase and enhance the population of anti-inflammatory Tregs while simultaneously downregulating the activation of pro-inflammatory cells. COYA 302 is being researched and evaluated to treat ALS.
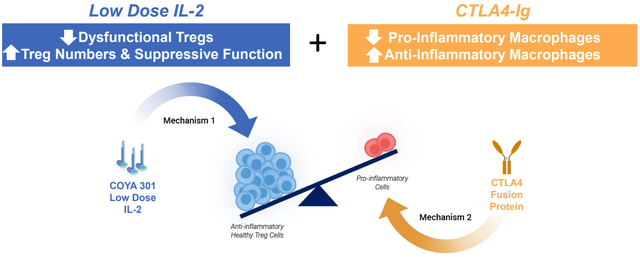
COYA 302 MoA Source (Company presentation )
Exhibit 9:COYA 302 MoA Source: Company Presentation
Proof of Concept Study and Development Timeline
Coya Therapeutics has completed a proof of concept (POC) study evaluating the safety, tolerability, Treg function, serum biomarkers of inflammation, and preliminary efficacy of COYA 301 in patients with Alzheimer's disease [AD]. The company plans to present the results of the study at the 2023 Keystone Symposia Meeting for Neurodegeneration in May 2023. Coya is also in the process of conducting IND-enabling studies to support the filing of an investigational new drug [IND] application to treat frontotemporal dementia.
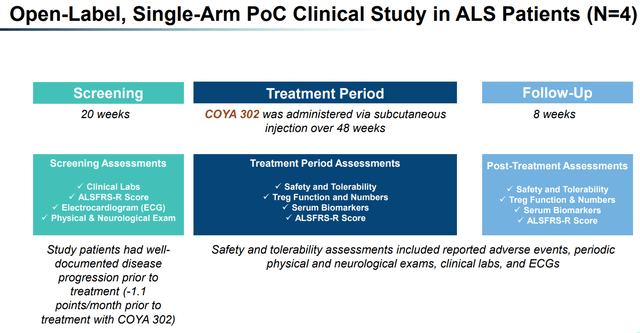
COYA 302 POC Study (Company presentation)
Exhibit 10: COYA 302 POC Study. Source: Company Presentation
The results from an open-label study of COYA 302, conducted by Dr. Appel and presented at the 2023 Muscular Dystrophy Association Clinical & Scientific Conference, showed promising outcomes for patients with amyotrophic lateral sclerosis (ALS). The study enrolled four patients who were treated with COYA 302 for 48 weeks and followed up for an additional eight weeks after treatment. The study assessments included the following:
Assessment of Functional Status via the Revised ALS Functional Rating Scale (ALSFRS-R): After 24 and 48 weeks of treatment, patients displayed either no deterioration or only minimal decline in functional status, compared to an average decrease of 1.1 points/ month in their ALSFRS-R score before starting the treatment. The average (±SD) ALSFRS-R scores at week 24 (33.75 ±3.3) and week 48 (32 ±7.8) following treatment initiation did not exhibit a significant difference when compared to the baseline ALSFRS-R score (33.5 ±5.9). This suggests a lack of decline in disease progression.
Treg Anti-inflammatory Function: Throughout the treatment duration, COYA 302 considerably enhanced Treg suppressive function, as evidenced by a notable percentage reduction in pro-inflammatory T-cell proliferation at 24 and 48 weeks relative to baseline. In particular, Treg suppressive function at 24 weeks (79.9±9.6) and 48 weeks (89.5±4.1) was significantly elevated compared to the baseline value (62.1±8.1).
Serum Biomarkers, Safety, and Tolerability: Consistent with enhanced Treg functionality, serum markers of inflammation, oxidative stress, and lipid peroxides demonstrated declines. COYA 302 exhibited good tolerability, and no serious adverse events were reported.

POC Study Results (Company presentation)
Exhibit 11: POC Study Results. Source: Company Presentation
Coya Therapeutics expects to initiate a Phase 2 randomized, double-blind, placebo-controlled clinical trial for COYA 302 in 120 patients with ALS and a clinical trial for COYA 301 in patients with frontotemporal dementia during the second half of 2023.
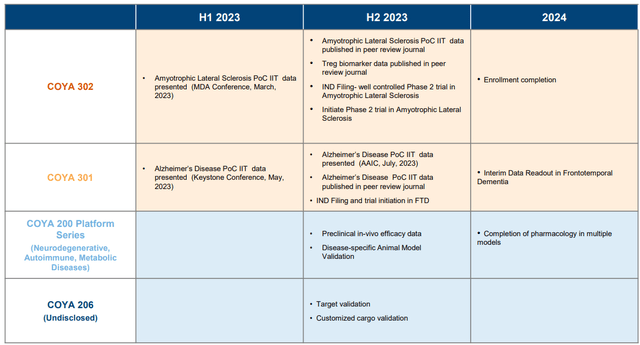
Timeline (Company presentation)
Exhibit 12: Timeline. Source: Company Presentation
ALS and FTD Market Overview
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia [FTD] are two closely linked neurodegenerative diseases, with 10% to 15% of patients diagnosed with FTD also being diagnosed with ALS. The basic difference between ALS and FTD is that ALS affects physical movement due to the death of motor neurons, while FTD impacts cognitive functions due to damage to the temporal and frontal lobes.
ALS, often referred to as Lou Gehrig's disease after the renowned baseball player who retired in 1939 due to the condition, is a rare disorder that attacks and destroys the nerve cells responsible for voluntary muscle movement. FTD is another neurological condition that leads to changes in behavior, personality, language, and motor skills, ultimately impairing a person's functionality. In the United States, approximately 5,000 individuals are diagnosed with ALS each year, with about 30,000 Americans presently living with the disorder. On the other hand, FTD affects an estimated 50,000 to 60,000 Americans and accounts for 10% to 20% of all dementia cases, making it one of the most common pre-senile dementias. The worldwide prevalence remains uncertain, with estimates among people aged 45 to 64 ranging between 15 and 22 per 100,000.
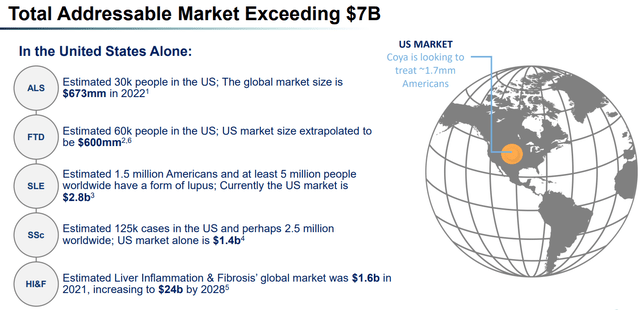
Total Addressable Market (Company presentation)
Exhibit 13: Total Addressable Market. Source: Company Presentation
While ALS primarily affects voluntary muscles, causing weakness and eventual paralysis, FTD is characterized by a progressive decline in behavior and/ or language, with memory generally preserved. Both conditions impact individuals' lives significantly as they progress over time and affect patients' social and occupational functioning. Although these disorders manifest differently, they are both progressive neurological life-threatening conditions with significant impacts on the lives of affected individuals and their families.
ALS Treatment Landscape
The complex, elusive, and progressive nature of ALS makes it particularly challenging for researchers to develop effective treatment options. While there isn’t any cure for ALS, the FDA has approved three new drugs in the past five years, taking the total tally to four FDA-approved drugs for ALS. These drugs claim to slow the progression of the disease and extend survival by a few months.
Drug | Company | Year Approved | Clinical Result |
Rilutek | Generic | 1995 | Extended median survival by 3 months. |
Radicava | Mitsubishi Tanabe Pharma | 2017 | At week 24, the changes in ALSFRS-R scores were -6.35 ± 0.84 for the placebo group (n=99) and -5.70 ± 0.85 for the Radicava group (n=100). |
Relyvrio | Amylyx Pharma. | 2022 | During the 24-week study, participants receiving the drug experienced a monthly decline of 1.24 points, while those on placebo declined by 1.66 points per month on the ALSFRS-R scale. |
Qalsody | Biogen/Ionis | 2023 | In the 28-week VALOR study, Qalsody-treated participants experienced a smaller, non-statistically significant decline in ALSFRS-R scores (adjusted mean difference of 1.2) compared to the placebo group. |
Exhibit 14: FDA-approved ALS drugs. Source: Own Research
The FDA granted accelerated approval to Biogen and Ionis' Qalsody to treat ALS patients with a SOD1 gene mutation based on its ability to reduce neurofilament light, a nerve injury biomarker. However, full approval was not granted due to a Phase 3 trial failure. The 2022 approval of Relyvrio also faced questions and skepticism from the scientific community due to a lack of strong evidence in relation to the effectiveness of the drug. Relyvrio was approved under the FDA’s accelerated pathway based on limited phase 2 study results. A more extensive phase 3 trial is underway, enrolling 664 patients with top-line results expected in 2024
Comparing these results to that of Coya’s proof of concept (POC) study, COYA 302 showed no or minuscule decline as measured by ALSFRS-R when compared to the baseline at 24 weeks (33.75 points at 24 weeks vs. 33.5 points at baseline). Although direct comparisons are certainly not feasible, these results instill confidence in the potential efficacy of COYA 302. The notable enhancement in Treg suppressive function throughout the treatment duration and the reduced serum markers of inflammation and oxidative stress further support the drug's potential effectiveness. Additionally, it should be noted that there are multiple other ALS drugs currently in clinical and pre-clinical trials.
Relyvrio, commercialized in late 2022, has set the yearly cost of treatment at $158,000, while the yearly cost of Radicava treatment is estimated at approximately $145,000. Based on these numbers and a 30,000-patient population size, the total market can be estimated at approximately $2.25 billion ($75,000 x 30,000), assuming an average of six months of treatment. The FTD and ALS indications both remain the company’s primary focuses and form a total patient population size of approximately 90,000, just in the U.S., who might significantly benefit from the company’s drug in the future.
Financials
After the conclusion of its initial public offering in January 2023, Coya Therapeutics is estimated to have a cash balance of about $20 million, which may suffice for 2023 and the first half of 2024. However, the company may need to raise more funds in the future, potentially undergoing multiple rounds of financing that could result in considerable dilution.
Risks
I foresee two predominant risks which encompass general or sector-specific aspects, specifically, the clinical development risk and the potential funding and dilution issues. Moreover, given Coya's micro-cap categorization, it is essential for investors to exhibit circumspection due to elements such as liquidity limitations and amplified market volatility.
Clinical Development Risk: Autoimmune and neurodegenerative diseases pose significant challenges due to their complex nature and lack of effective treatment options. We still lack considerable comprehensive knowledge pertaining to these diseases, limiting our ability to develop successful treatment options. The rate of clinical failures for disease-modifying treatments (DMTs) aimed at slowing or halting disease progression has been more than 95% for major neurodegenerative disorders. For ALS, the success probability for drugs in Phase 3 to launch is a mere 4%, and for those currently under Phase 1 or pre-clinical trials, it is even less.
Capital and Dilution Risk: Coya Therapeutics’ existing liquidity status allows the commencement of COYA 301 and 302 clinical trials; however, advancing other candidates through the pipeline will require raising additional capital, resulting in dilution. The degree of dilution hinges on the materialization of positive short- and long-term catalysts that might bolster the company's market value.
Concluding Statements and Catalysts
- The recent positive findings from the COYA 302 POC study and the initial results of its Treg cell therapy highlight Coya Therapeutics' potential to transform the treatment landscape for numerous neurodegenerative and autoimmune disorders.
POC clinical data in AD generated from a study at Houston Methodist Hospital; presentation in May and July 2023; peer-reviewed publication in 2023.
COYA 301 for FTD: IND submission and Phase 1 initiation (H2 2023); interim data (H2 2023) and COYA 302 for ALS: IND filing and Phase 1 start (H2 2023). COYA 201 for NDD and autoimmune disease: Animal model studies completion ( H1 2023). COYA 206 for undisclosed indications: target validation (H1 2023).
COYA 101 for ALS: Phase 2b initiation through grant funding or a partnership (2024).
- I believe Coya Therapeutics embodies a high-risk, very high-reward proposition that is well worth considering.
Editor's Note: This article covers one or more microcap stocks. Please be aware of the risks associated with these stocks.
This article was written by
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours.
Business relationship disclosure: Coya Therapeutics is a client of Quantum Media Group, LLC
Seeking Alpha's Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.
