Pfizer, GSK, AbbVie among pharmas set to rise with May catalysts
sitox
With the first trading day in May coming on Monday, many pharmaceutical companies will be hoping their returns fare better than they did in April.
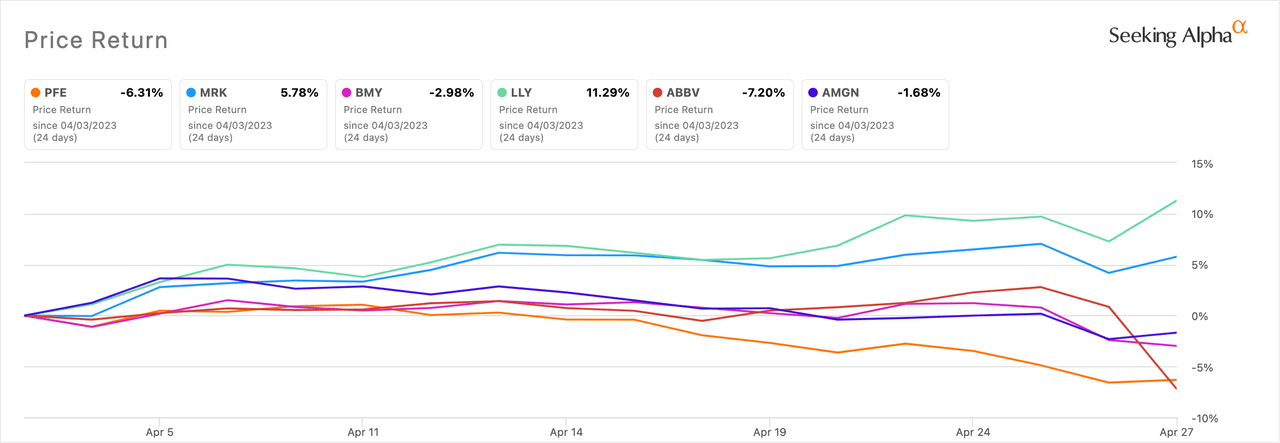
The iShares U.S. Pharmaceuticals ETF (IHE) returned a paltry 1.6% in April. The ETF's top two holdings are Johnson & Johnson (NYSE:JNJ) and Merck (NYSE:MRK), which account for, respectively, 23.6% and 20.7% of the fund.
The best performing US large pharma or biotech in the month was Eli Lilly (LLY), turning in an impressive 11.3% return. Despite a Q1 2023 bottom line miss, the pharma was buoyed by weight loss data for its diabetes drug Mounjaro (tirzepatide).
AbbVie (NYSE:ABBV) was the worst performer with a -7.2% return. It was negatively impacted by disappointing Q1 results.
Several companies that would like to forget April have a chance to redeem themselves in May as there are major events, such as US FDA action dates on drug or biologic applications, that could provide a share price boost.
May 3 could bring a smile to the faces of GlaxoSmithKline (GSK) management and stockholders as that's the date the FDA is slated to render a decision on its respiratory syncytial virus (RSV) vaccine Arexvy. In March, a panel of FDA advisors unanimously recommended its approval.
SVB Securities analyst Geoffrey Porges has previously said the RSV vaccine market could reach $10B by 2030.
Pfizer (NYSE:PFE) will also hear from the FDA in May about its own RSV shot, Abrysvo. Agency advisors also recommended Abrysvo. Both Pfizer (PFE) and GSK (GSK) are gearing up to launch the jabs before the end of the year.
Eyenovia (EYEN) is expecting a decision on May 8 on its resubmitted New Drug Application for MydCombi ophthalmic spray for mydriasis. The following day, a decision is expected for Protalix Biotherapeutics' (PLX) resubmitted Biologics License Application for pegunigalsidase alfa for Fabry disease.
An FDA advisory committee will meet May 12 to discuss Sarepta Therapeutics' (SRPT) BLA for SRP-9001 for Duchenne muscular dystrophy. The action date is May 29. Sarepta (SRPT) stock was hit earlier this month after a report that some FDA staff opposed the gene therapy.
On May 19, the FDA is expected to act on Krystal Biotech's (KRYS) B-VEC for dystrophic epidermolysis bullosa. A decision is expected the same day on an NDA for obeticholic acid as a treatment for pre-cirrhotic liver fibrosis due to nonalcoholic steatohepatitis from Intercept Pharmaceuticals (ICPT).
AbbVie (ABBV) will find out on May 21 the fate of its epcoritamab BLA for relapsed/refractory large B-cell lymphoma. The company is partnered with Genmab (GMAB) on the candidate.
An FDA decision on an additional indication for Blueprint Medicine's (BPMC) Ayvakit (avapritinib) for indolent systemic mastocytosis is expected May 22. The following day, a decision of ImmunityBio's (IBRX) Anktiva (N-803) for BCG-unresponsive non-muscle-invasive bladder cancer carcinoma is planned.
The final catalyst of May is likely on May 29 with a decision on Innoviva's (INVA) sulbactam-durlobactam for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia.
Sometime during the month, the FDA is slated to act on Lexicon Pharmaceuticals' (LXRX) sotagliflozin for heart failure.