Vericel Corporation: A Promising Future In Advanced Cell Therapies

Summary
- The FDA's recent approval of NexoBrid for the treatment of burns is expected to drive revenue growth in 2023.
- Vericel's MACI autologous cell therapy product for knee cartilage defects is on track for a potential accelerated commercial launch in 2024.
- Vericel's strong financial position and positive industry trends make it an attractive investment opportunity for the long term.
Wongsakorn Napaeng/iStock via Getty Images
Vericel Corporation (NASDAQ:VCEL) is a biopharmaceutical company specializing in advanced cell therapies for severe burn care and sports medicine. Vericel has an impressive portfolio of FDA-approved products, allowing them to capitalize on the growing healthcare sector that demands regenerative therapies. Vericel Corporation is an innovative leader with a strong pipeline of FDA-approved and investigational products that address unmet medical needs. The company's focus on regenerative therapies for sports medicine and severe burn care has resulted in a diverse product portfolio with significant revenue potential. MACI, the company's flagship product, has shown impressive revenue growth and represents a unique solution for patients suffering from cartilage defects in the knee. The recent approval of NexoBrid for the removal of eschar in severe thermal burns in adults and the anticipated launch of arthroscopic MACI in 2024 further reinforces the company's strong growth potential. With a solid balance sheet and no debt, Vericel is well-positioned to continue delivering strong financial and operational results. Overall, Vericel Corporation presents an attractive investment opportunity in the burgeoning regenerative medicine industry.
Leading Products Provide Strong Growth
Cell therapy pioneer, Vericel Corporation, has announced remarkable financial outcomes for the final quarter of 2022. The firm's aggregate net revenue rose by 11%, reaching $52.7 million in comparison to the corresponding period in the previous year. This notable accomplishment is particularly striking given the difficulties presented by the recent economic turbulence.
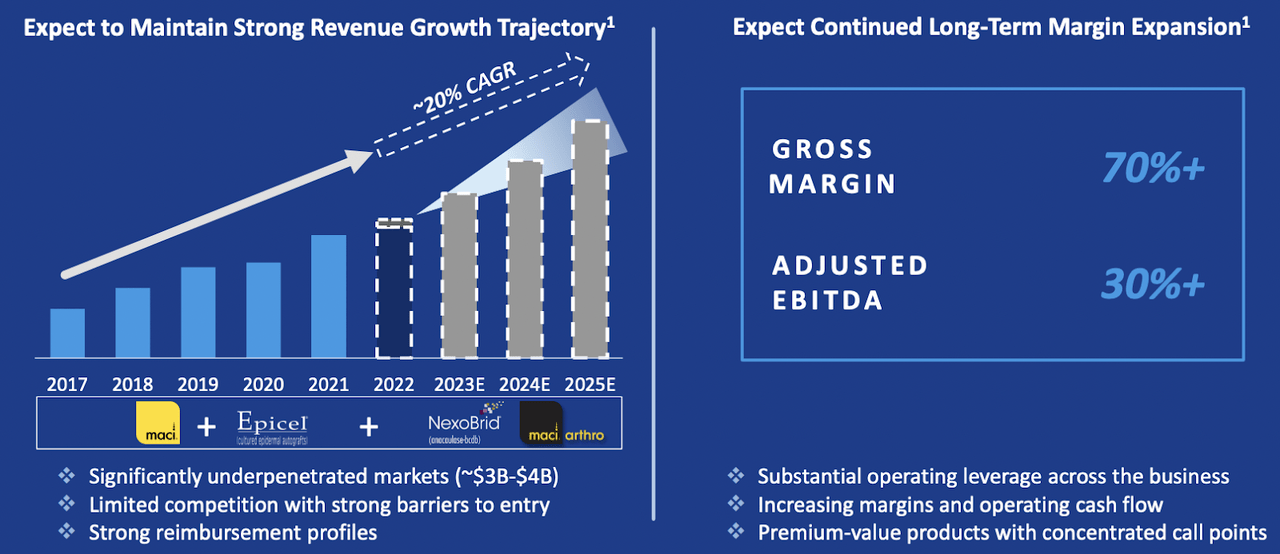
The primary catalyst for revenue enhancement has been Vericel's leading product, MACI, which achieved a net revenue of $46.3 million in the fourth quarter. This represents a 24% growth year-over-year. Additionally, the company experienced a sequential surge of about 50% relative to the prior quarter, marking the highest quarterly revenue since MACI's introduction. These numbers reflect the escalating demand for MACI.
In the fourth quarter of 2022, Vericel's gross margin stood at 73%, illustrating the firm's capacity to preserve profitability even as revenues expand. The net income for the quarter amounted to $5.9 million, or $0.12 per diluted share, signifying a 31% increment compared to the prior year.
ycharts.com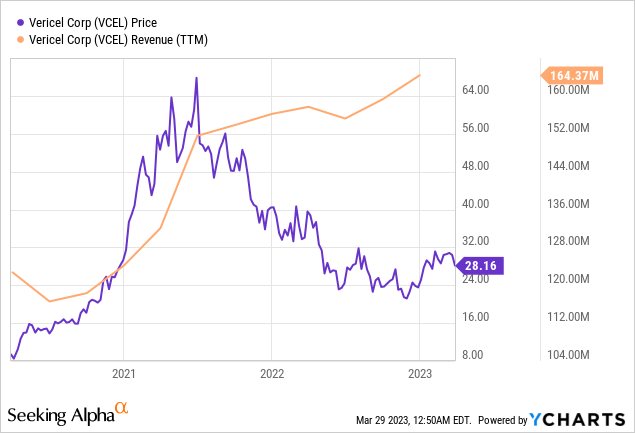
The firm's financial projection for 2023 appears optimistic, with anticipated total net revenue ranging between $180 and $188 million, and expected MACI revenue falling between $152 and $156 million. The company also envisions future revenues will benefit from an upcoming hastened commercial introduction of arthroscopic MACI.
Mature Pipeline With Future Potential
Vericel Corporation currently has three FDA-approved products with two being fully commercialized.
Investors Presentation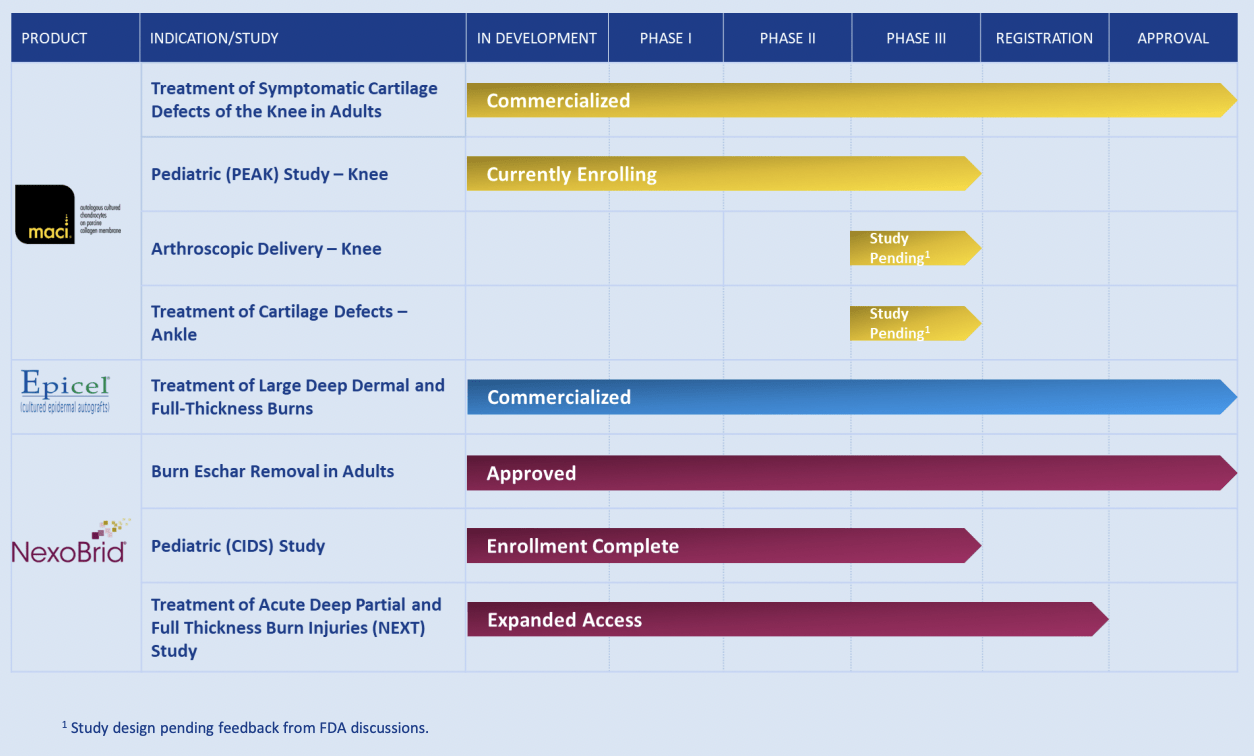
The first product, MACI, utilizes the patient's own cartilage cells to mend adult knee cartilage defects. It is delivered through an implantable membrane which takes on the shape and size of the defect before it is surgically implanted into the joint.
The second product, Epicel, is composed of the patient's skin cells to form sheets of epidermis that substitute lost skin due to strong burns. This product has been approved for both adults and pediatric patients who suffer from deep dermal or full-thickness burns covering at least 30% of the body surface area.
NexoBrid constitutes a biological product that uses a bromelain-based enzymatic solution to eliminate eschar for deep full and partial-thickness thermal burns in adults. It selectively dissolves the eschar without damaging underlying viable tissue when applied topically to the affected area.
Recent Business Updates
In 2022, Vericel Corporation made remarkable strides in advancing its product pipeline and extending its business reach. The firm recently revealed FDA approval for NexoBrid. The product is slated for commercial availability in the US during the second quarter of 2023. Additionally, Vericel has made headway in the progression of MACI. The company intends to launch a human factors validation study to facilitate the extension of the MACI label, encompassing arthroscopic application of MACI for addressing knee cartilage defects. Vericel foresees an expedited potential commercial debut of arthroscopic MACI in 2024.
The advancements made by Vericel are heartening, and the robust performance of MACI at the end of the year signals a bright outlook. The company's dedication to broadening its product range and securing FDA approval for novel therapies showcases its resolve to deliver inventive solutions for patients. The projected acceleration of total revenue growth for 2023 and 2024 attests to Vericel's capability to implement its business plan and seize market opportunities.
Product-Related Mitigating Factors
While MACI represents an innovative approach to cartilage repair, there are potential concerns related to its mechanism of action. First, the use of autologous cells introduces variability in cell quality between patients, which could impact graft success. Donor site morbidity, including pain and cartilage damage at the site of cell harvest, is also a concern. Additionally, the use of a porcine collagen scaffold introduces the potential for immunogenic responses, which could lead to inflammation or graft rejection. Moreover, the long-term viability and functionality of the implanted chondrocytes remain uncertain, and there may be concerns regarding the integration of the graft with the surrounding native tissue.
Epicel's autologous nature is advantageous in reducing the risk of graft rejection; however, several challenges are associated with its mechanism of action. The process of expanding epidermal cells in vitro could lead to cellular senescence or phenotypic changes, impacting graft quality. Additionally, the need for a biopsy site also introduces the risk of donor site complications.
Despite the potential benefits of NexoBrid in facilitating eschar removal, certain limitations are associated with its enzymatic approach. The potential for over-debridement or damage to healthy surrounding tissue during the process is a concern. Additionally, the efficacy and safety of NexoBrid have not been established for certain types of burns, such as chemical or electrical burns, or for burns on the face, perineum, genitalia, or in circumferential burns.
The Competitive Landscape
Vericel Corporations' flagship products, MACI and Epicel, offer superior efficacy and safety profiles compared to their competitors in the market. MACI is a first-in-class product that uses the patient's own chondrocytes to repair the damage. The product has demonstrated superior clinical outcomes compared to microfracture and mosaicplasty procedures, which are commonly used for treating cartilage defects. MACI has been shown to improve pain, function, and quality of life in patients for up to 5 years post-treatment. In contrast, competing products such as DeNovo NT and Carticel, which also use autologous chondrocytes, have more limited clinical data.
Similarly, Epicel has been shown to provide superior wound healing and scar reduction compared to conventional treatments such as split-thickness skin grafting. Epicel also has a lower risk of infection, rejection, and scarring than allogeneic skin grafts, which are derived from donor skin. Competing products such as ReCell have not demonstrated comparable clinical outcomes or cost-effectiveness.
NexoBrid, Vericel's latest product approved by the FDA, offers several advantages over conventional surgical debridement, which is a painful and time-consuming procedure that can cause bleeding, infection, and scarring. NexoBrid selectively dissolves the eschar without harming the viable tissue underneath, reducing the need for repeat surgeries and improving wound healing and cosmetic outcomes. Competing products such as Santyl have not shown comparable efficacy.
Looking Ahead
In conclusion, Vericel Corporation has established a strong position in the cell therapy market with its innovative products. The company's flagship product, MACI, has shown significant revenue growth and clinical efficacy in treating symptomatic, full-thickness cartilage defects of the knee. The recent approval of NexoBrid for the removal of eschar in thermal burns and the planned launch of arthroscopic MACI for cartilage defects in the knee and ankle are expected to drive further revenue growth and market penetration in 2023 and beyond. Vericel's focus on quality, safety, and innovation, as well as its strong financial position with no debt and ample cash reserves, position the company for continued success in the highly competitive and evolving biopharmaceutical industry. Moreover, the company's commitment to expanding its pipeline and pursuing strategic partnerships and acquisitions is likely to create additional value for its shareholders in the long term. Overall, Vericel Corporation appears to be a promising investment opportunity for those seeking exposure to the growing field of regenerative medicine and advanced therapies.
This article was written by
Analyst’s Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Seeking Alpha's Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.