Iovance up 13% on completing Biologics License Application submission for lifileucel
Mar. 24, 2023 6:22 PM ETIovance Biotherapeutics, Inc. (IOVA)By: Jonathan Block, SA News Editor1 Comment
Nasekom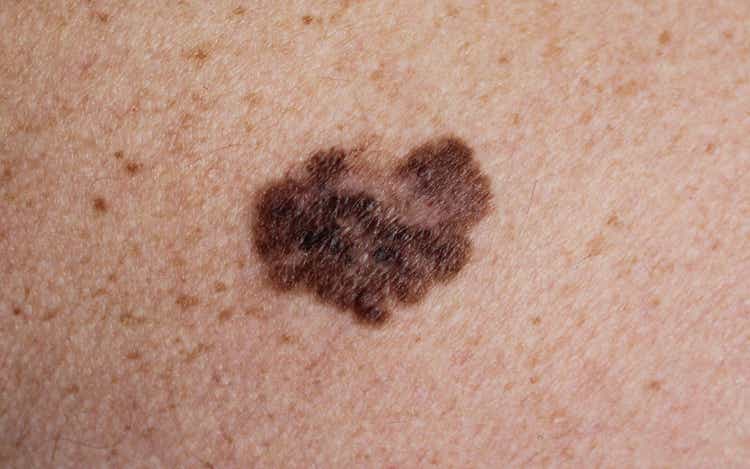
- Iovance Biotherapeutics (NASDAQ:IOVA) is up 13% in after-hours trading Friday after reporting it has completed its rolling Biologics License Application for lifileucel for melanoma.
- Lifileucel, a tumor infiltrating lymphocyte therapy, is under investigation for patients with advanced melanoma that have failed on anti-PD-1/L1 therapy and targeted therapy.
- There are no approved treatments for melanoma in this setting.
- Read why Seeking Alpha contributor BioSci Capital Partners reiterated a strong buy rating for Iovance (IOVA) in February.