Competition Coming For AbbVie's Blockbuster Humira

Summary
- It’s among the prohibitively expensive biologic pharmaceuticals being replaced by cheaper “biosimilar” copycats.
- Relief is on the way for patients who need Humira, a product made by AbbVie.
- Several drugmakers are expected to begin offering biosimilar versions of Humira in the coming months, including: Bristol Myers Squibb, GSK, Johnson & Johnson, and Merck.
Hailshadow
By Andrew Prochnow
Worldwide sales of Humira have totaled $200 billion in the last 20 years—just about the biggest haul in the history of prescription drugs. The bonanza was predicated on charging as much as $84,000 for a year’s supply.
But price relief is on the way for patients who need Humira, a product made by AbbVie (ABBV) to combat conditions ranging from arthritis and psoriasis to Crohn’s disease and ulcerative colitis.
They won’t be paying as much for their medication because Humira belongs to a class of high-priced pharmaceuticals known as biologics, which drug companies are replacing with cheaper substitutes.
The stand-ins, called biosimilars, cost an average of 27% less than the originals they mimic, according to the National Institutes of Health, a government research agency. The savings mount up because of the sky-high cost of biologics.
Unlike most pharmaceuticals, which are synthetic, biologic drugs are produced or extracted from humans, animals, plants or bacteria. Biosimilar drugs resemble their reference drug (i.e., the brand-name biologic) in structure and function, but they’re not exact copies.
The relationship between biologics and biosimilars approximates the connection between brand-name synthetic drugs and their generic substitutes.
Here come the biosimilars
Three factors are converging to cause the switch from biologics to biosimilars: Biologic patents are expiring, biosimilar makers are suing biologic companies and the government has taken action to lower drug prices.
Adalimumab, the biologic often sold under the brand name Humira, provides a classic example of biosimilars’ downward pressure on prices, and shows how it came about.
The patent on Humira doesn’t expire until 2034, but competition’s about to heat up because of litigation brought by biosimilar makers and because the Affordable Care Act of 2010 provides an easier path for biosimilars to win U.S. Food and Drug Administration (FDA) approval.
A biosimilar version of Humira was scheduled to begin reaching the market in January, the GoodRx Health website says. As many as seven other substitutes could become available in the second half of this year.
But what’s up with these copycats?
Center for Drug and Research, U.S. Food and Drug Administration 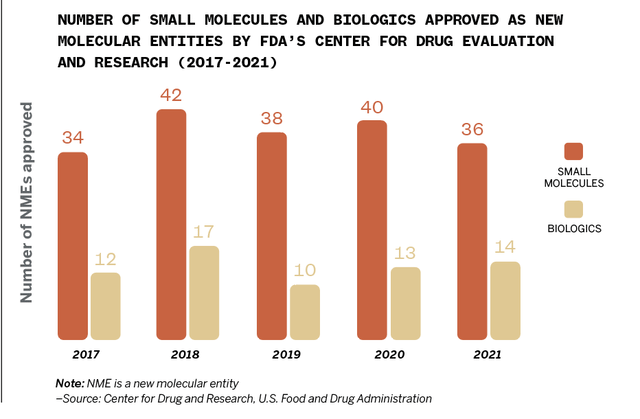
Biologics and biosimilars
Because they’re biological, biologics vary more than synthesized pharmaceuticals in form, function or efficacy. As biological entities, biologics are also more sensitive than synthetic drugs to environmental conditions during production, storage and use.
To accommodate those differences, regulators subject biologics and biosimilars to stricter standards than synthesized pharmaceuticals.
Approved substitutes for traditional synthetic pharmaceuticals go by the name “generics.” While biosimilars can pinch hit for biologics, they’re not the same as generics, notes Brandon Shank, a clinical pharmacy specialist at the University of Texas MD Anderson Cancer Center.
“Generic products are simple molecules, while biosimilars are large proteins or macromolecules, so they are different in their structure and how they are produced,” Shank told ASH Clinical News.
To earn the biosimilar designation, a drug must be safe, effective and work in much the same way as the brand-name reference drug. And, like generics, biosimilars are more affordable than the drugs they replace.
However, generic medications typically cost up to 85% less than their brand-name equivalents, quite a bit less than the average 27% difference between biologics and their biosimilar replacements.
Yet, because biologics qualify for the specialty drug class of medications that cost more than $1,000 a month, any reduction in price becomes meaningful. Increased market penetration of biosimilars can help rein in the expense.
The jaw-dropping $7,000 monthly cost of Humira, for example, shows why specialty drugs account for only 2% of prescriptions in the United States but nearly 50% of total drug revenue.
The biosimilars market
The seven drugmakers expected to begin offering biosimilar versions of Humira in the coming months will be selling copies of adalimumab, the active ingredient. The companies are Amgen (AMGN), Organon (OGN), Coherus (CHRS), the Sandoz Division of Novartis (NVS), Pfizer (PFE), Viatris (VTRS) and privately held Boehringer Ingelheim.
Three more manufacturers may join them next year—Alvotech (ALVO), Fresenius (FMS) and privately held Celltrion.
Biosimilars have been available in Europe for nearly 15 years but have been used on a limited basis in the U.S. for about seven years. The FDA has approved 621 biologics, and 40 of them have associated biosimilars on the market.
Besides adalimumab (aka Humira), biosimilars that are available include the nearly unpronounceable lineup of bevacizumab, epoetin alfa, etanercept, filgrastim, infliximab, insulin glargine, pegfilgrastim, ranibizumab, rituximab and trastuzumab.
In addition to AbbVie, the world’s top manufacturers of biologics in terms of annual sales include Amgen (AMGN), Bristol Myers Squibb (BMY), GSK (GSK), Johnson & Johnson (JNJ), Merck (MRK), Novartis (NVS), Novo Nordisk (NVO), Ono Pharmaceutical (OTCPK:OPHLY), Pfizer (PFE), Regeneron (REGN), Roche (OTCQX:RHHBY), Sanofi (SNY) and Teva (TEVA).
So-called middlemen that negotiate prices and distribute biosimilars— including healthcare and insurance provider Cigna (CI) and CVS Health (CVS), the drugstore chain and insurer—also stand to benefit financially from the proliferation of the substitute pharmaceuticals, The Wall Street Journal says.
Consumers win, too. Lower prices inevitably make cutting-edge treatments more widely available, observes Steven Newmark, chief legal officer for the Global Healthy Living Foundation.
“With so many biosimilars coming on to the market, we hope it signals a sea change that biosimilars will finally live up to their intended purpose of providing effective, safe treatment at a more affordable cost in the coming years,” Newmark told Healio Rheumatology, a healthcare publication.
Editor's Note: This article discusses one or more securities that do not trade on a major U.S. exchange. Please be aware of the risks associated with these stocks.
This article was written by
Disclosure: I/we have no stock, option or similar derivative position in any of the companies mentioned, and no plans to initiate any such positions within the next 72 hours. Business relationship disclosure: This article was written for Luckbox magazine by a contributor.

