Top Searches
- News
- India News
- CDSCO grants EUA to Bharat Bio's intranasal Covid-19 vaccine
CDSCO grants EUA to Bharat Bio's intranasal Covid-19 vaccine
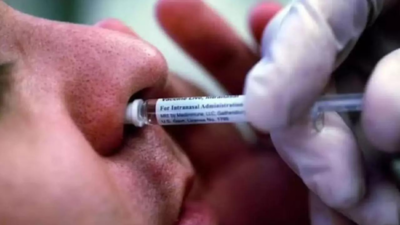
HYDERABAD: In what is the world’s first intranasal Covid-19 vaccine to be granted emergency use authorisation (EUA), the Indian drug regulator on Tuesday approved Bharat Biotech’s intranasal vaccine BBV154 for primary immunisation of those aged 18 years and above.
The Central Drugs Standard Control Organisation (CDSCO) has granted approval to BBV154 for restricted use in emergency situation, Union health minister Dr Mansukh Mandaviya announced via a tweet on Tuesday.
“Big Boost to India’s Fight Against COVID-19! Bharat Biotech’s ChAd36-SARS-CoV-S COVID-19 (Chimpanzee Adenovirus vaccine) recombinant nasal vaccine approved by @CDSCO_INDIA_INF for primary immunization against COVID-19 in 18+ age group for restricted use in emergency situation,” the minister tweeted.Mandaviya said the move will further strengthen the collective fight against the pandemic. “India has harnessed its science, R&D, and human resources in the fight against COVID-19 under PM @NarendraModi Ji’s leadership. With the science-driven approach & Sabka Prayas, we will defeat COVID-19,” he added.
Watch Bharat Biotech's nasal vaccine gets CDSCO nod for emergency use authorisation
The Central Drugs Standard Control Organisation (CDSCO) has granted approval to BBV154 for restricted use in emergency situation, Union health minister Dr Mansukh Mandaviya announced via a tweet on Tuesday.
“Big Boost to India’s Fight Against COVID-19! Bharat Biotech’s ChAd36-SARS-CoV-S COVID-19 (Chimpanzee Adenovirus vaccine) recombinant nasal vaccine approved by @CDSCO_INDIA_INF for primary immunization against COVID-19 in 18+ age group for restricted use in emergency situation,” the minister tweeted.Mandaviya said the move will further strengthen the collective fight against the pandemic. “India has harnessed its science, R&D, and human resources in the fight against COVID-19 under PM @NarendraModi Ji’s leadership. With the science-driven approach & Sabka Prayas, we will defeat COVID-19,” he added.
Watch Bharat Biotech's nasal vaccine gets CDSCO nod for emergency use authorisation
FOLLOW US ON SOCIAL MEDIA
FacebookTwitterInstagramKOO APPYOUTUBE
Start a Conversation
end of article









