Australia provisionally approves Moderna's COVID-19 shot for children under 5
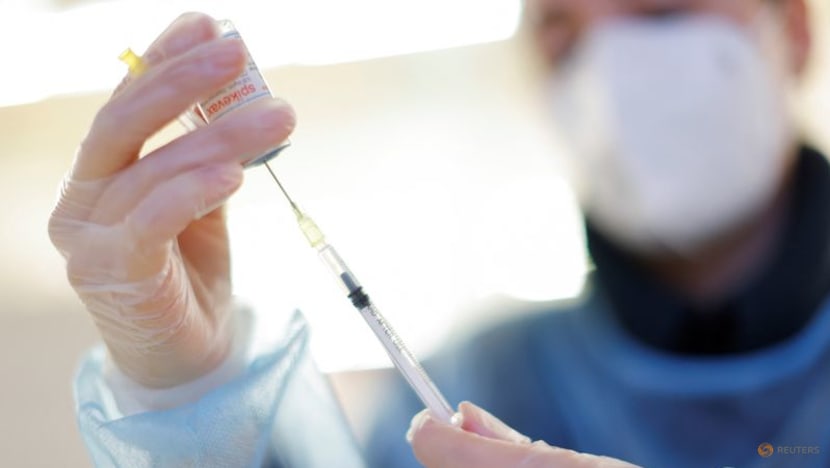
A nurse prepares a booster dose of the Moderna COVID-19 vaccine, Spikevax, at a vaccination centre in Berlin on Jan 1, 2022. (File photo: Reuters/Michele Tantussi)
Moderna said late on Monday (Jul 18) that Australia's drug regulator, the Therapeutic Goods Administration (TGA), had provisionally approved its mRNA COVID-19 vaccine, Spikevax, for use in children between six months old and five years old.
The shot had earlier been provisionally approved in the country for individuals aged six years and older and as a booster dose for those aged 18 years and older, TGA said in a separate statement.
The move comes as Australia battles a major virus outbreak fuelled by the highly transmissible new Omicron subvariants, BA.4 and BA.5, with hospital admissions surpassing record levels in several states.
Authorities in the country expect millions of new infections and are urging people to wear masks indoors, although they have ruled out any tough curbs to contain the spread.
Last week, Argentina and Canada also approved Moderna's COVID-19 vaccine for children aged six months to five years.
BOOKMARK THIS: Our comprehensive coverage of the COVID-19 pandemic and its developments
Download our app or subscribe to our Telegram channel for the latest updates on the coronavirus outbreak: https://cna.asia/telegram