- News
- City News
- vadodara News
- Vadodara: Kit for early detection of cervical cancer
Vadodara: Kit for early detection of cervical cancer
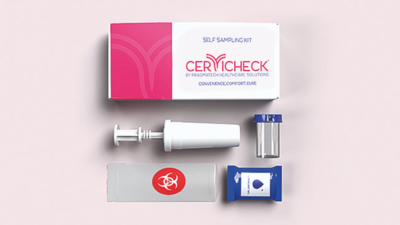
Self-sampling kit
VADODARA: Every year, over 60,000 women in the country lose their lives to cervical cancer even as the World Health Organisation (WHO) has called for global elimination of the malaise by 2030. A major reason is late detection of the disease and especially because women are reluctant to undergo the process of sample collection at the hospital.
Thanks to the efforts of a city-based startup, women can now collect the samples for testing themselves even from the comfort of their homes.
Pragmatech Healthcare Solutions – a startup floated by Anirban Palit, Sayantani Pramanik,Palna Patel and Dr Bhagirath Modi – is testing a self-sampling kit Cervicheck.The kit can be used by women at any place or can even be used in camps or similar settings to screen women for the Human Papillomavirus that is responsible for more than 95 per cent of cervical cancer cases.
Palit worked for around a decade in the in vitro diagnostic (IVD) industry while his wife Pramanik worked in the research and development field of leading pharma companies in the country. Patel is a prominent lawyer and social worker ,while Modi is a veteran gynaecologist.
Palit said that while self-sampling kits are available abroad for screening purposes to test women for HPV, Cervicheck can collect samples sufficient for further testing too. “Many women do not return for triage tests if they are found positive for HPV. With the sample collected using our kits, the triage tests can also be conducted,” he said.
Patel pointed out that women will need longer feel embarrassed as they can collect the samples at whenever and wherever they are comfortable. “They can do so at home and we are looking to ensure that the samples are collected from their residences and tested in a laboratory. The startup is collaborating with a chain laboratory for the purpose of doorstep collection of samples and their testing.
Palit said that while they bootstrapped Rs 15 lakh funds, they got an aid of Rs 30 lakh from the Venture Center at Pune, Rs 20 lakh from the HDFC Smart-up CSR Grant and other grants from various sources. The startup is not limiting itself to self-sampling kits alone, but has also started work on a screening assay after receiving a Rs 50 lakh Biotechnology Industry Research Assistance Council (BIRAC) Ignition Grant.
“The assay will be a point of care test that can detect pre-cancerous cells,” said Palit.
The self-sampling kit is the first indigenously developed kit in India which has received an approval from the Central Drugs Standard Control Organisation (CDSCO) for ap ivotal clinical evaluation. It is undergoing evaluation at SSG Hospital,Vadodara and Prayas Amrita, Pune. Palit said that the trials are expected to get over in July.
They are targeting a launch in January next year.
Thanks to the efforts of a city-based startup, women can now collect the samples for testing themselves even from the comfort of their homes.
Pragmatech Healthcare Solutions – a startup floated by Anirban Palit, Sayantani Pramanik,Palna Patel and Dr Bhagirath Modi – is testing a self-sampling kit Cervicheck.The kit can be used by women at any place or can even be used in camps or similar settings to screen women for the Human Papillomavirus that is responsible for more than 95 per cent of cervical cancer cases.
Palit worked for around a decade in the in vitro diagnostic (IVD) industry while his wife Pramanik worked in the research and development field of leading pharma companies in the country. Patel is a prominent lawyer and social worker ,while Modi is a veteran gynaecologist.
Palit said that while self-sampling kits are available abroad for screening purposes to test women for HPV, Cervicheck can collect samples sufficient for further testing too. “Many women do not return for triage tests if they are found positive for HPV. With the sample collected using our kits, the triage tests can also be conducted,” he said.
Patel pointed out that women will need longer feel embarrassed as they can collect the samples at whenever and wherever they are comfortable. “They can do so at home and we are looking to ensure that the samples are collected from their residences and tested in a laboratory. The startup is collaborating with a chain laboratory for the purpose of doorstep collection of samples and their testing.
Palit said that while they bootstrapped Rs 15 lakh funds, they got an aid of Rs 30 lakh from the Venture Center at Pune, Rs 20 lakh from the HDFC Smart-up CSR Grant and other grants from various sources. The startup is not limiting itself to self-sampling kits alone, but has also started work on a screening assay after receiving a Rs 50 lakh Biotechnology Industry Research Assistance Council (BIRAC) Ignition Grant.
“The assay will be a point of care test that can detect pre-cancerous cells,” said Palit.
The self-sampling kit is the first indigenously developed kit in India which has received an approval from the Central Drugs Standard Control Organisation (CDSCO) for ap ivotal clinical evaluation. It is undergoing evaluation at SSG Hospital,Vadodara and Prayas Amrita, Pune. Palit said that the trials are expected to get over in July.
They are targeting a launch in January next year.
FOLLOW US ON SOCIAL MEDIA
FacebookTwitterInstagramKOO APPYOUTUBE
Looking for Something?

Start a Conversation
end of article
Visual Stories
Quick Links
UP NewsDelhi TemperatureChennai WeatherBangalore TemperatureUP Board Result 2022Coronavirus in DelhiRTPCR test in GurgaonHyderabad RainPollution level in BangaloreDelhi SmogDelhi TemperatureNoida AQIGurgaon AQI todayFire in MumbaiMumbai RainsCovid 19 RT PCR Test in NoidaDelhi AQI todaySrinagar encounter










