Top Searches
- News
- India News
- Coronavirus live updates: Pfizer files for US authorization of antiviral pill
Coronavirus live updates: Pfizer files for US authorization of antiviral pill
Drugmaker Pfizer Inc has signed a deal with a UN-backed group to allow other manufacturers to make its experimental Covid-19 pill, a move that could make the treatment available to more than half of the world's population. Stay with TOI for live updates:Read Less
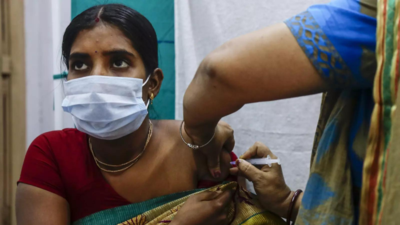
India at lowest Covid threat level: US
“Your risk of contracting Covid may be lower if you are fully vaccinated with an FDA-authorised vaccine,” said a US travel advisory for India issued on Monday. The CDC website stated: “Key Information for travellers to India: Make sure you are fully vaccinated before traveling to India. Travellers should follow recommendations or requirements in India, including wearing a mask". Read full story
FDA promises quick review of Pfizer booster for all adults, CDC meets Friday, reports Reuters
New York City will allow crowds into Times Square on New Year's Eve to ring in 2022, Mayor Bill de Blasio announced Tuesday, after the pandemic muted last year's celebrations, reports AFP
India resumes vaccine exports to 4 countries
Myanmar, Bangladesh, Nepal and Iran have been the first recipients of resumed vaccine exports from India almost eight months after they were halted due to rising cases of Covid-19. The government had stopped vaccine exports as the brutal second wave hit India in April-May this year. It’s only after India had administered close to a billion doses of vaccines did the government relax restrictions. Commercial contracts were also kept in abeyance as production was procured for domestic use. Read full story
Cases surge in new COVID hot spots of Michigan, Minnesota in US, reports AP
Italy tightens Covid rules on public transport, reports IANS
Roche terminates partnership with Atea to develop Covid-19 pill, reports Reuters
Pfizer agrees to let other companies make its Covid-19 pill, reports AP
Pfizer files for US authorization of promising Covid-19 antiviral pill, reports Reuters
Lawsuits challenging Biden workplace vaccine rule sent to 6th Circuit, reports Reuters
US pharmaceutical company Pfizer on Tuesday asked regulators to authorize its Covid19 pill after it was shown to cut hospitalization or death by nearly 90 per cent among newly-infected high risk patients, reports AFP
