Moderna Drops After FDA Setback on Teen Covid Shots
(Bloomberg) -- Moderna Inc. lost more than $7 billion in market value on Monday after its Covid-19 shot failed to secure U.S. regulatory authorization in teenagers.
The Food and Drug Administration wants more time to weigh international analyses of the risks of a rare heart inflammation, myocarditis, which may not be completed before the end of the year, Moderna said in a statement on Sunday. The stock fell 5.1% to $327.79 at 12 p.m. in New York.
“The delay should not be a surprise,” and should also push back timing of the company’s filing for an emergency nod in even younger children, Morgan Stanley analyst Matthew Harrison wrote in a note to clients. It also puts the distribution of Moderna’s shots well behind Pfizer Inc. and BioNTech SE which were authorized for children as young as 12 in May.
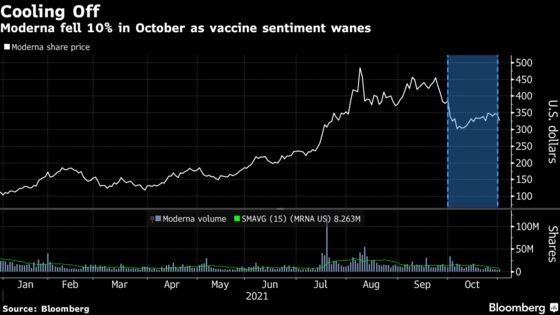
The stock slumped 10% in October -- the worst month for the company since March -- after the successes of Merck & Co.’s Covid-19 pill put a damper on vaccine stock enthusiasm. Still, Moderna has more than tripled this year and is the year’s best performer in the S&P 500 by a long shot after its landmark inclusion in the benchmark gauge.
Specialist investors still see Moderna as overvalued now that the Covid-19 crisis is slowing down, Jared Holz a strategist with Oppenheimer, said in an interview. The size of the potential market for booster shots next year and beyond also remains up for debate.
“Moderna has been validated in a significant way but the effect that just the Covid-19 vaccine is having as an upside driver has been diminishing lately. Health-care investors would like to see the pipeline beyond coronavirus,” Holz said.
©2021 Bloomberg L.P.