European Commission inks new pact with Novavax for potential COVID-19 vaccine
Under the new contract, the member states will be able to buy up to 100 million doses of the Novavax vaccine, with an option for 100 million additional doses over the course of 2021, 2022, and 2023
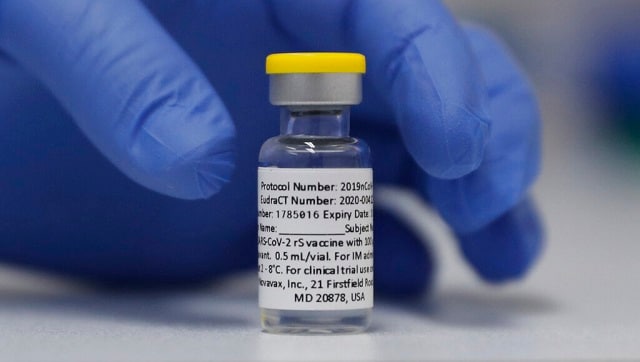
File Image of a vial of the Phase 3 Novavax coronavirus vaccine ready for use in the trial at St. George's University hospital in London. AP
The European Commission on Thursday approved its seventh Advanced Purchase Agreement (APA) with pharmaceutical company Novavax to ensure access to a potential vaccine against COVID-19 in Q4 of 2021 and in 2022.
in Q4 of 2021 and in 2022.
Under this contract, the member states will be able to purchase up to 100 million doses of the Novavax vaccine, with an option for 100 million additional doses over the course of 2021, 2022, and 2023. The member states will also be able to donate vaccines to lower and middle-income countries or to re-direct them to other European countries.
The latest contract with Novavax complements an already broad portfolio of vaccines to be produced in Europe, including the contracts with AstraZeneca, Sanofi-GSK, Janssen Pharmaceutica NV, BioNtech-Pfizer, CureVac, Moderna and the concluded exploratory talks with Valneva. The contract is a key step towards ensuring that Europe is well prepared to face the COVID-19 pandemic.
pandemic.
“As new coronavirus variants are spreading in Europe and around the world, this new contract with a company that is already testing its vaccine successfully against these variants is an additional safeguard for the protection of our population. It further strengthens our broad vaccine portfolio, to the benefit of Europeans and our partners worldwide,” said president of the European Commission, Ursula von der Leyen.
variants are spreading in Europe and around the world, this new contract with a company that is already testing its vaccine successfully against these variants is an additional safeguard for the protection of our population. It further strengthens our broad vaccine portfolio, to the benefit of Europeans and our partners worldwide,” said president of the European Commission, Ursula von der Leyen.
As variants are spreading, we need to stay vigilant.
We approved a new contract with @Novavax for 200 million doses of its vaccine, which is already being tested successfully against variants.
With our broad vaccine portfolio we protect Europeans and help vaccinate the world. pic.twitter.com/3IQ2692CAz
— Ursula von der Leyen (@vonderleyen) August 4, 2021
Stella Kyriakides, Commissioner for Health and Food Safety, said: “Vaccinations in the EU are advancing and we are closer to our target of 70% fully vaccinated citizens by the end of summer. Our new agreement with Novavax expands our vaccine portfolio to include one more protein-based vaccine, a platform showing promise in clinical trials. We will continue working tirelessly to ensure that our vaccines continue to reach citizens in Europe and around the world, to end the pandemic as quickly as possible.”
What is APA or Advance Purchase Agreements?
The European Commission presented on 17 June a European strategy to accelerate the development, manufacturing and deployment of effective and safe vaccines against COVID-19 . In return for the right to buy a specified number of vaccine doses in a given timeframe, the Commission finances part of the upfront costs faced by vaccines producers in the form of Advance Purchase Agreements.
. In return for the right to buy a specified number of vaccine doses in a given timeframe, the Commission finances part of the upfront costs faced by vaccines producers in the form of Advance Purchase Agreements.
In view of the current and new escape SARS-CoV-2 variants, the Commission and the member states are negotiating with companies already in the EU vaccine portfolio new agreements that would allow them to purchase adapted vaccines in sufficient quantities to reinforce and prolong immunity.
As per the APA, the Commission will enter into agreements with individual vaccine producers on behalf of member states. In return for the right to buy a specified number of vaccine doses in a given timeframe and at a given price, part of the upfront costs faced by vaccines producers will be financed from the ESI. This will be done in the form of APAs.
These agreements are negotiated with individual companies according to their specific needs and with the aim of supporting and securing an adequate supply of vaccines. They de-risk the necessary investments related to both vaccine development and clinical trials and the preparation of the at-scale production capacity along the entire vaccine production chain which is required for rapid deployment of sufficient doses of an eventual vaccine in the EU and globally.
The conditions of the contract reflect the balance between the prospect of the producer providing a safe and effective vaccine quickly and the investment needed to deploy the vaccine on the European market. The contracts with companies are concluded through a procurement process run by the Commission on behalf of all participating member states. The budgetary authorities, the European Parliament and the Council have made €2.7 billion available for the purpose.
The aim of the negotiations is to conclude APAs with individual companies under the best possible conditions.
What is the overall efficacy of Novavax COVID-19 vaccine?
vaccine?
On 14 June, Novavax had announced that NVX-CoV2373, its recombinant nanoparticle protein-based COVID-19 vaccine, demonstrated 100 percent protection against moderate and severe disease, 90.4 percent efficacy overall, and met the primary endpoint in its PREVENT-19 pivotal Phase 3 trial.
vaccine, demonstrated 100 percent protection against moderate and severe disease, 90.4 percent efficacy overall, and met the primary endpoint in its PREVENT-19 pivotal Phase 3 trial.
The study enrolled 29,960 participants across 119 sites in the US and Mexico to evaluate the efficacy, safety, and immunogenicity, with an emphasis on recruiting a representative population of communities and demographic groups most impacted by the disease.
"Today, Novavax is one step closer to addressing the critical and persistent global public health need for additional COVID-19 vaccines. These clinical results reinforce that NVX-CoV2373 is extremely effective and offers complete protection against both moderate and severe COVID-19
vaccines. These clinical results reinforce that NVX-CoV2373 is extremely effective and offers complete protection against both moderate and severe COVID-19 infection," said Stanley C Erck, President and Chief Executive Officer, Novavax. "Novavax continues to work with a sense of urgency to complete our regulatory submissions and deliver this vaccine, built on a well understood and proven platform, to a world that is still in great need of vaccines."
infection," said Stanley C Erck, President and Chief Executive Officer, Novavax. "Novavax continues to work with a sense of urgency to complete our regulatory submissions and deliver this vaccine, built on a well understood and proven platform, to a world that is still in great need of vaccines."
Upon regulatory approvals, the company said Novavax remains on track to reach a manufacturing capacity of 100 million doses per month by the end of the third quarter and 150 million doses per month by the end of the fourth quarter of 2021.
"PREVENT-19 confirms that NVX-CoV2373 offers a reassuring tolerability and safety profile," said Gregory M Glenn, M.D., President of Research and Development, Novavax. "These data show consistent, high levels of efficacy and reaffirm the ability of the vaccine to prevent COVID-19 amid ongoing genetic evolution of the virus. Our vaccine will be a critical part of the solution to COVID-19
amid ongoing genetic evolution of the virus. Our vaccine will be a critical part of the solution to COVID-19 and we are grateful to the study participants and trial staff who made this study possible, as well as our supporters, including the US Government."
and we are grateful to the study participants and trial staff who made this study possible, as well as our supporters, including the US Government."
In the placebo-controlled, observer-blinded study randomized 2:1, NVX-CoV2373 demonstrated overall efficacy of 90.4 percent (95 percent CI: 82.9, 94.6), achieving its primary endpoint. Seventy-seven cases were observed: 63 in the placebo group and 14 in the vaccine group. All cases observed in the vaccine group were mild as defined by the trial protocol. Ten moderate cases and four severe cases were observed, all in the placebo group, yielding a vaccine efficacy of 100 percent (95 percent CI: 87.0, 100) against moderate or severe disease.
Efficacy endpoints were accrued from January 25 through April 30, 2021 — a time when the Alpha (B.1.1.7) variant, first identified in the UK, became the predominant strain in the US. Other strains, including Variants of Interest (VoI) and Variants of Concern (VoC), were also on the rise during the PREVENT-19 endpoint accrual window.
NVX-CoV2373 also showed success among "high-risk" populations (defined as over age 65, under age 65 with certain comorbidities, or having life circumstances with frequent COVID-19 exposure): vaccine efficacy was 91.0 percent (95 percent CI: 83.6, 95.0), with 62 COVID-19
exposure): vaccine efficacy was 91.0 percent (95 percent CI: 83.6, 95.0), with 62 COVID-19 cases in the placebo group and 13 COVID-19
cases in the placebo group and 13 COVID-19 cases in the vaccine group.
cases in the vaccine group.
also read

COVID-19: India records 39,097 new cases in last 24 hrs, takes total to 3.1 crore; recovery rate at 97,3%
India's total toll due to the coroanvirus rose to 4,20,016 with 546 more deaths since Friday. The number of active cases went up by 3,464 to 4,08,977.
Is the Delta variant making younger adults 'sicker, quicker’?
Physicians said almost all patients coming in this are unvaccinated, is younger and mainly in their 20s or 30s.

Over 3.09 crore unutilised vaccine doses available with states and private hospitals, says Centre
It said that more than 45.37 crore vaccine doses have been provided to states and UTs so far through all sources and a further 59,39,010 doses are in the pipeline