.
Text Dimension:
.
.
.
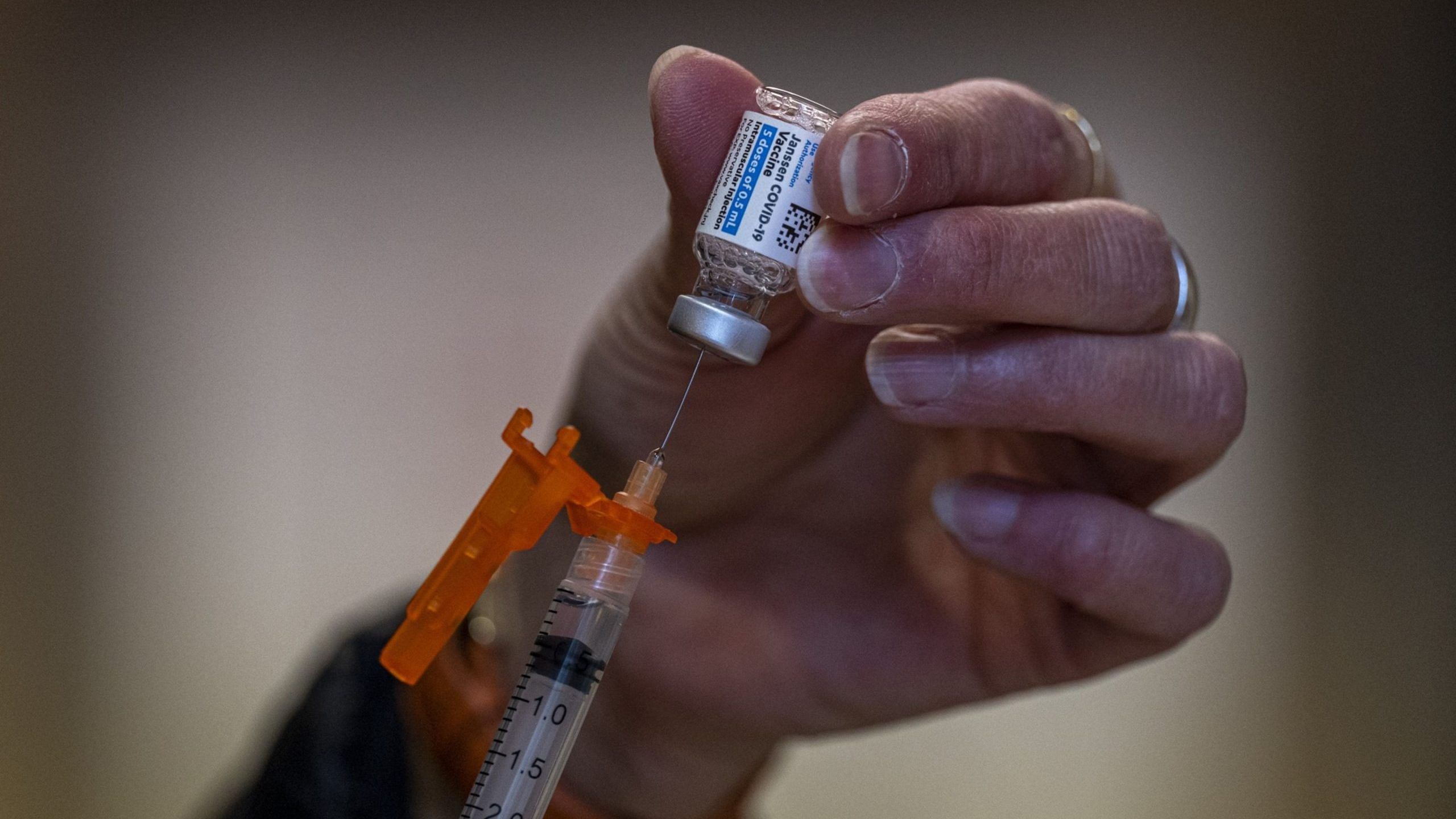
New Delhi: United States pharma firm Johnson & & Johnson recorded significant contamination dangers at a Baltimore biodefence plant 7 months prior to a cross-contamination event messed up 15 million dosages of its Covid injection, the Washington Message reported Thursday.
The event, initial reported on 31 March, took place at a plant in Baltimore, Maryland, where employees unintentionally blended active ingredients of Johnson & & Johnson and also AstraZeneca injections.
The plant is run by Emergent BioSolutions, a production companion to both Johnson & & Johnson and also AstraZeneca, the latter of which is yet to obtain authorization for using its injection in the United States.
A team record by a federal government oversight board launched Wednesday stated Emergent was “conscious” of the issues however “stopped working to take adequate activity”.
Though Emergent has actually taken on much objection for current gaps, papers divulged by a Legislature panel Wednesday disclosed that Johnson & & Johnson carried out a digital audit of the center in June2020 The audits located mold and mildew, poor gowning and also wipe-down treatments and also “lacking” contamination-control actions. On 24 July 2020, the audit searchings for were sent out to Emergent.
The Baltimore event postponed future deliveries of Johnson & & Johnson dosages in the United States and also abroad.
Talking to press reporters previously this month, United States Chargé D’Affaires in Delhi Daniel Smith stated while Washington is worried concerning the “human disaster” in India, it is not yet in a placement to send out readymade dosages of injections– generated by AstraZeneca and also Johnson and also Johnson– that are existing extra in the nation.
This is since they are still being evaluated by the FDA, he stated.
In August 2020, the Trump management stated it will certainly spend $1 billion in an injection established by Johnson & & Johnson, guaranteeing 100 million dosages.
100 million J&J injection dosages on hold
Emerging BioSolutions Chief Executive Officer Robert G. Kramer has stated greater than 100 million dosages of Johnson & & Johnson’s injection are currently on hold as regulatory authorities inspect them for feasible contamination.
In late April, the firm briefly closed down procedures at the plant at the demand of the United States Fda (FDA) and also recognized it requires to “bring back self-confidence” in its job.
While affirming prior to a Legislature subcommittee Wednesday, Kramer stated the center is anticipated to return to job “in an issue of days”, which the company approved “complete duty” of the current gaps.
Head Of State Joe Biden introduced Monday the United States will certainly export 80 million injection dosages– 60 million dosages of the AstraZeneca stab that are waiting for USFDA authorization and also an additional 20 million various other Covid injections that are accepted however are existing extra. Nevertheless, it is still vague the number of of those dosages will certainly pertain to India.
Sign up for our networks on YouTube & & Telegram
Why information media remains in dilemma & & Exactly how you can repair it
India requires complimentary, reasonable, non-hyphenated and also doubting journalism much more as it deals with several dilemmas.
Yet the information media remains in a dilemma of its very own. There have actually been harsh discharges and also pay-cuts. The very best of journalism is diminishing, accepting unrefined prime-time phenomenon.
ThePrint has the finest young press reporters, writers and also editors benefiting it. Maintaining journalism of this top quality requires clever and also assuming individuals like you to spend for it. Whether you stay in India or overseas, you can do it right here