Reviva: Developing Next-Generation Antipsychotic Drug
Schizophrenia is the most common psychotic disease with a global prevalence in about 1% of the population, affecting approximately 3.5 million people in the U.S. and 20 million globally. Despite the high prevalence, there is currently no therapy that adequately addresses the complex mental disorder.
Current antipsychotics prescribed for schizophrenia are plagued with suboptimal efficacy, poor tolerability, adverse effects and abnormally high dropout rates.
As a result of suboptimal efficacy, the major symptoms of schizophrenia, such as chronic mood symptoms and cognitive dysfunction, are not adequately addressed to date. In addition, approved antipsychotics are reported to cause adverse effects at varying degrees including extrapyramidal symptoms (EPS), akathisia, obesity, diabetes, cholesterol, and sexual dysfunction.
Due to the myriad adverse effects, patients tend to discontinue treatment. It is estimated that the discontinuation rates are in the range of 30% to 50% in the short-term treatment of acute patients and 42% to74% in the long-term treatment of stable schizophrenia patients.
Reviva (NASDAQ: RVPH), a clinical Phase 3 stage pharmaceutical company, is currently focused on developing novel therapies for respiratory and neuropsychiatric diseases, including schizophrenia. Its lead drug candidate aims to address this huge unmet need with its novel drug candidate brilaroxazine (RP5063).
About Reviva’s Lead Drug Candidate
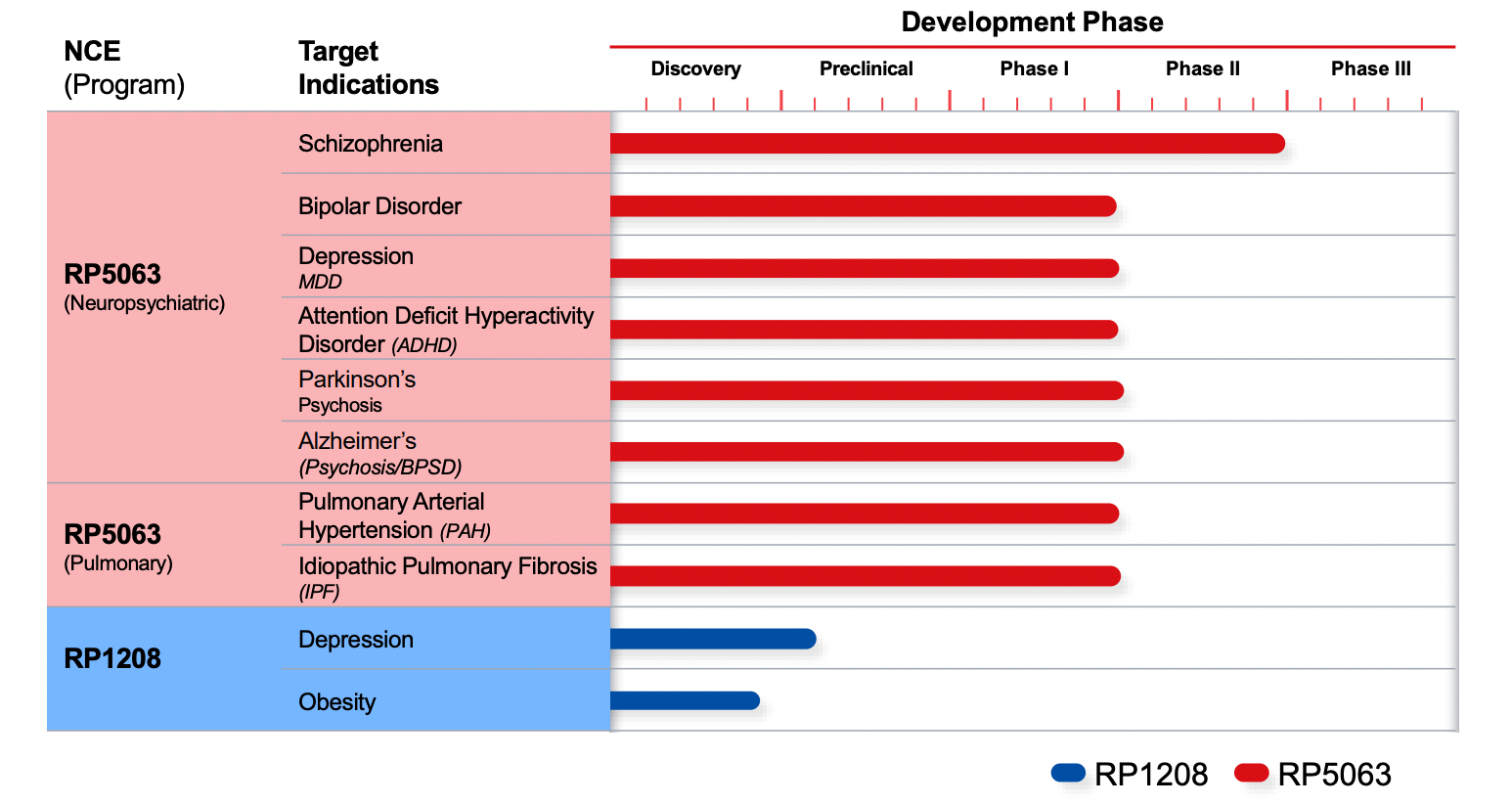
Brilaroxazine is Reviva’s lead asset. It has demonstrated efficacy as well as safety for the treatment of schizophrenia in Reviva’s Phase 2 clinical trials in over 230 patients.
Brilaroxazine sets itself apart from other drug candidates in the space due to its unique receptor activities as it can potentially treat multiple neuropsychiatric diseases, such as schizophrenia, bipolar disorder, depression, ADHD, as well as psychosis and behavioral symptoms in Alzheimer’s / Dementia (BPSD), Parkinson’s psychosis. Brilaroxazine is also being developed to treat interstitial lung diseases, pulmonary arterial hypertension (PAH), and idiopathic pulmonary fibrosis (IPF).
It is worth noting that, unlike most antipsychotic drugs that are associated with brain disease, Reviva’s drug would work even for lung complications due to its serotonin signaling specificity that is implicated in the pathobiology of both mental illness and lung diseases.
About Reviva
Reviva is currently developing a portfolio of internally discovered next-generation innovative therapies using integrated chemical genomic-driven approaches and proprietary chemistries for diseases of significant unmet needs and large market size.
The company has multiple products in the pipeline at various stages of development for chronic conditions in large therapeutic areas and gained a substantial patent position in the U.S. and worldwide.
Reviva is led by CEO, President and Founder, Dr. Laxminarayan Bhat, who has over 20 years of experience in drug discovery and development. Prior to founding Reviva, he held research positions at XenoPort, ARYx Therapeutics, and Higuchi Biosciences Center in the U.S. Dr. Bhat has authored over 25 research papers published in peer-reviewed scientific journals and is an inventor on more than 100 granted patents.
The global addressable market size for brilaroxazine is massive with the company targeting six indications each valued at billions of dollars:
- $7.9 billion for schizophrenia by 2021
- $5.4 billion for bipolar disorder by 2024
- $15.9 billion for depression by 2023
- $24.9 billion for ADHD by 2025
- $14.6 billion for PAH by 2026
- $5.9 billion for IPF by 2023
That’s a cumulative market size of over $74 billion.
Reviva is poised to start its Phase 3 pivotal study for schizophrenia and Phase 2 studies for PAH and IPF this year. It has filed an S-1 to raise $23 million to fund the Phase 3 schizophrenia study and anticipates providing topline data readouts within 18 months (Q4 2022).
The Phase 3 study is underpinned by positive Phase 2 clinical trial results reported in April. The Phase 2 study assessed the safety and efficacy of brilaroxazine in 234 subjects with acute exacerbation of schizophrenia or schizoaffective disorder, brilaroxazine met its primary endpoint, which was a reduction in total Positive and Negative Syndrome Scale (PANSS) at the end of the treatment from baseline versus placebo.
The drug candidate also met all safety endpoints including clinical, labs, body weight, prolactin, lipids, fasting glucose, and EKG. The PANSS total score was reduced by 20 points, a statistically significant treatment difference from the placebo. Brilaroxazine also mitigated positive symptoms and negative symptoms and improved social functioning and cognition.
Importantly, the FDA has agreed to consider a potential ‘Superior Safety' label claim, if there is a positive outcome on a relevant endpoint in the upcoming pivotal Phase 3 clinical study in schizophrenia.
To learn more about Reviva, you can visit its website here.
Reviva Pharmaceuticals (NASDAQ: RVPH) is a partner of Benzinga. The information in this article does not represent the investment advice of Benzinga or its writers.
© 2021 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.



