Top Searches
- News
- City News
- mumbai News
- BMC floats global EoI to buy 50L vaccines, 1st local body to do so
BMC floats global EoI to buy 50L vaccines, 1st local body to do so
Representative image
MUMBAI: The BMC on Wednesday floated a global expression of interest (EoI) to buy 50 lakh vaccines (1 crore doses, considering two doses per person) in a bid to speed up its inoculation drive.
The EoI, officials said, was floated in a record time of 24 hours after the civic body got a green signal from the state government to buy its own vaccines.
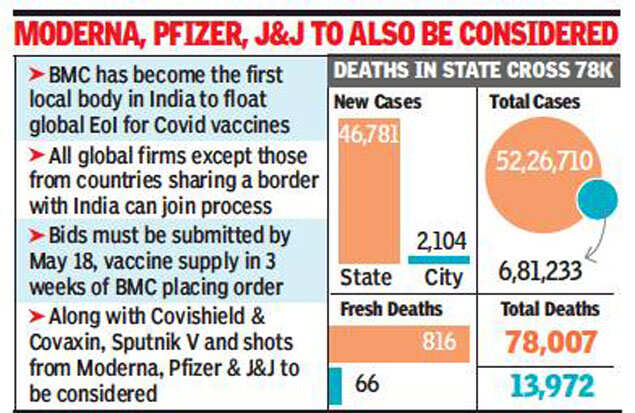
The BMC has said vaccines must be supplied in three weeks after it places the order. Companies from across the world, except those from countries sharing a border with India, can take part in the process. This means Chinese vaccine-makers are barred.
While several state governments such as those in Maharashtra, Uttar Pradesh, Odisha and Karnataka have taken an in-principle decision to invite global tenders and procure vaccines, so far no tender or EoI has been floated by any of them. The BMC, the richest municipal corporation in Asia, is the first local body in India to float a global EoI to buy vaccines. Officials said interested suppliers will have to submit their bids by May 18, and they will be opened the same evening.
‘City needs fewer doses, will get quicker response’
BMC officials said they are open to a deal with any approved vaccine manufacturer that comes forward to provide the 1 crore doses for which a global expression of interest has been floated. Apart from Covaxin and Covishield, the civic body will consider Sputnik V, which was developed in Russia and accorded emergency use authorisation in India, and vaccines developed by Moderna, Pfizer and Johnson & Johnson.
BMC’s decision to float an EoI may seem at odds with the state’s own plans, but officials said Mumbai’s needs being fewer, the chances of getting a quicker response from manufacturers were higher. While the state may seek supplies of up to 4 crore doses, BMC chief I S Chahal said Mumbai could do with1crore doses to ensure vaccination for all. More than 25 lakh doses have been given in the metropolis so far to various sections, including healthcare workers, senior citizens and thosein the18+ age-group.
“The applicant should have a valid licence to manufacture/ export Covid–19 vaccine to India as per specifications mentioned in the EoI from the Competent Authority and/or Food and Drug Administration, government of India. The vaccine to be supplied must be as per the guidelines issued by Indian Council for Medical Research (ICMR). DCGI approval for supply of the vaccines in India (is also needed). The applicant, besides having own manufacturing licence, should hold a valid WHO GMP certificate issued by the licensing authorities for all the premises from where vaccine is being manufactured,” the EoI stated, outlining the eligibility criteria.
The EoI, officials said, was floated in a record time of 24 hours after the civic body got a green signal from the state government to buy its own vaccines.

The BMC has said vaccines must be supplied in three weeks after it places the order. Companies from across the world, except those from countries sharing a border with India, can take part in the process. This means Chinese vaccine-makers are barred.
While several state governments such as those in Maharashtra, Uttar Pradesh, Odisha and Karnataka have taken an in-principle decision to invite global tenders and procure vaccines, so far no tender or EoI has been floated by any of them. The BMC, the richest municipal corporation in Asia, is the first local body in India to float a global EoI to buy vaccines. Officials said interested suppliers will have to submit their bids by May 18, and they will be opened the same evening.
‘City needs fewer doses, will get quicker response’
BMC officials said they are open to a deal with any approved vaccine manufacturer that comes forward to provide the 1 crore doses for which a global expression of interest has been floated. Apart from Covaxin and Covishield, the civic body will consider Sputnik V, which was developed in Russia and accorded emergency use authorisation in India, and vaccines developed by Moderna, Pfizer and Johnson & Johnson.
BMC’s decision to float an EoI may seem at odds with the state’s own plans, but officials said Mumbai’s needs being fewer, the chances of getting a quicker response from manufacturers were higher. While the state may seek supplies of up to 4 crore doses, BMC chief I S Chahal said Mumbai could do with1crore doses to ensure vaccination for all. More than 25 lakh doses have been given in the metropolis so far to various sections, including healthcare workers, senior citizens and thosein the18+ age-group.
“The applicant should have a valid licence to manufacture/ export Covid–19 vaccine to India as per specifications mentioned in the EoI from the Competent Authority and/or Food and Drug Administration, government of India. The vaccine to be supplied must be as per the guidelines issued by Indian Council for Medical Research (ICMR). DCGI approval for supply of the vaccines in India (is also needed). The applicant, besides having own manufacturing licence, should hold a valid WHO GMP certificate issued by the licensing authorities for all the premises from where vaccine is being manufactured,” the EoI stated, outlining the eligibility criteria.
FacebookTwitterLinkedinEMail
end of article
Top Stories Right Now
- india3.5 lakh fresh Covid cases, toll over 4.000 for 2nd day in a row
- indiaStop price gouging by private suppliers, India tells China
- indiaCovid live: Karnataka now has most active cases in India
- indiaIn ‘suitable cases’, go for house arrest, not jail: Supreme Court
- businessTax hikes in 2020 to blame for sharp rise in fuel prices now
