Effectiveness of Johnson & Johnson COVID Vaccine Explained Ahead of FDA Review
Today, a Food and Drug Administration (FDA) committee is meeting to discuss the emergency use authorization (EUA) application submitted by Johnson & Johnson for its single-dose COVID-19 vaccine candidate.
The FDA's Vaccines and Related Biological Products Advisory Committee will meet via teleconference between 9:00 a.m. ET and 5:30 p.m. ET to review the pharmaceutical company's EUA submission for the prevention of COVID-19 in adults aged 18 or over.
If all goes well, the FDA could authorize the vaccine as early as Saturday—in which case, it would become the third COVID-19 shot to be approved for emergency use in the United States. But what do we know about the effectiveness of the vaccine?
Documents released by the company and FDA ahead of the meeting analyzed results from a Phase III clinical trial of the vaccine involving around 44,000 people globally, showing that the shot provides high levels of protection against severe disease and death from COVID-19.
The documents show that the overall efficacy of the vaccine was around 66 percent against moderate to severe disease 28 days after vaccination—a figure that was consistent across age groups.
There were some differences in the efficacy of the vaccine depending on where trial participants were located around the world.
For example, efficacy of the shot against moderate to severe COVID-19 among trial participants in the United States was 72 percent. But in South Africa—where more than 90 percent of cases were caused by the more transmissible 501Y.V2 variant of concern—efficacy against moderate to severe disease was 64 percent.
The figure for efficacy against moderate to severe disease in South Africa provided in the documents is seven percentage points higher than suggested by data that the company released in late January.
Despite the lower South African figures, when it comes to protecting against severe COVID-19, the shot still had an efficacy of 82 percent.
And in the United States, this figure was 86 percent. Overall efficacy against severe disease across all countries and ages in the trial was 85 percent at least 28 days after vaccination.
According to the documents, the vaccine is particularly effective in the prevention of COVID-19 hospitalization and death. No COVID-19 related deaths were reported among trial participants who received the vaccine at least 28 days after the shot.
The documents also say preliminary data suggests that the vaccine provides strong protection against asymptomatic infections as well, although more data is needed to confirm this conclusion.
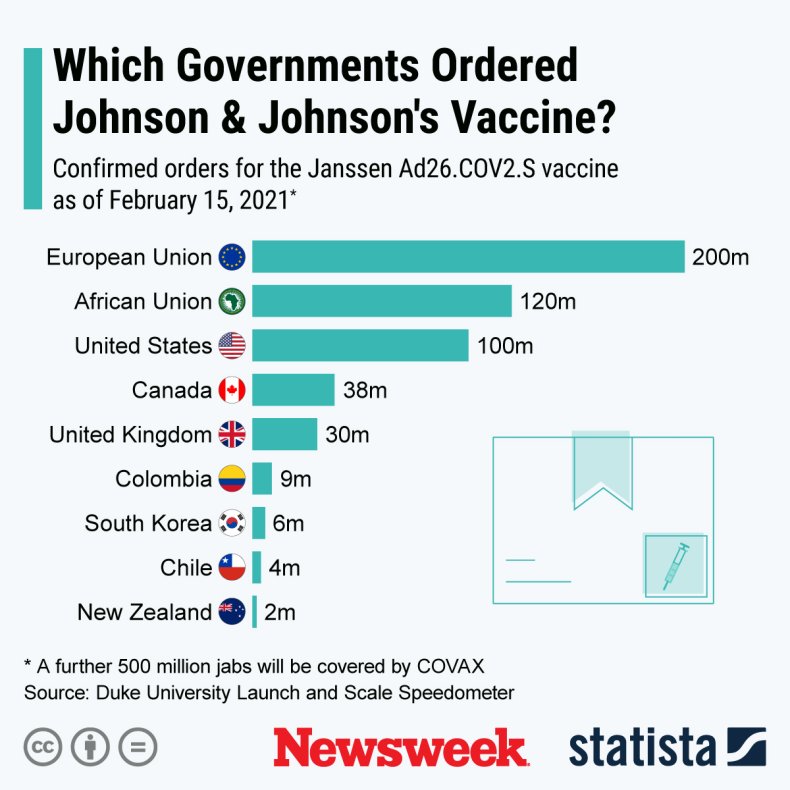
The graphic below, provided by Statista, shows confirmed orders for the Johnson & Johnson vaccine as of February 15, 2021.
Newsweek, in partnership with NewsGuard, is dedicated to providing accurate and verifiable vaccine and health information. With NewsGuard's HealthGuard browser extension, users can verify if a website is a trustworthy source of health information. Visit the Newsweek VaxFacts website to learn more and to download the HealthGuard browser extension.