Russia's Sputnik V vaccine is 91.6% effective against coronavirus when two doses are given, according to peer-reviewed interim results from its phase three trial.
Scientists said no serious side effects were associated with having the jab, and most reported adverse events were mild, including flu-like symptoms, pain at the injection site and weakness or low energy.
Kirill Dmitriev, chief executive of the Russian Direct Investment Fund (RDIF), said scientists had found Sputnik to be effective against the variant first detected in Kent, with further tests to take place on the South Africa and Brazil variants.
Sputnik V has been approved by 15 countries, including Argentina, Hungary and the United Arab Emirates and this number will rise to 25 by the end of next week, said Mr Dmitriev.
Russia has begun the process of submitting it to the European Medicines Agency (EMA) for approval in the European Union.
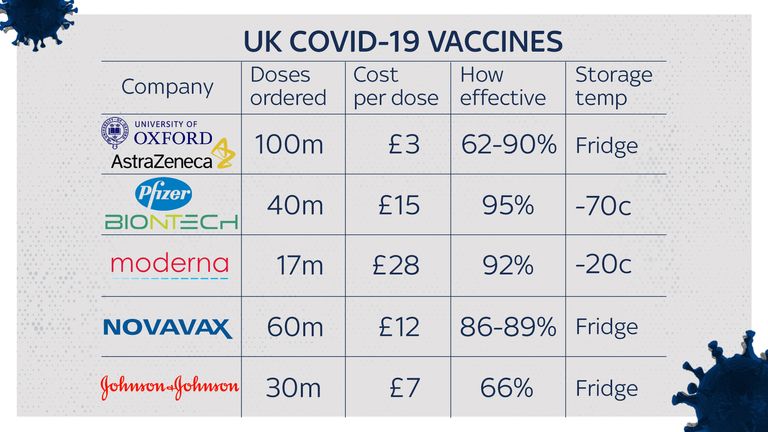
The funding body behind the inoculation has now said a combined two-stage dosage of the Sputnik vaccine and the Oxford/AstraZeneca vaccine could help in the fight against coronavirus mutations.
Mr Dmitriev told the BBC: "We generally believe that two shots of different vaccines - AstraZeneca and Sputnik - may actually work better because immunity gets stronger."
He said clinical trials to test the AstraZeneca/Sputnik theory were due to get under way.
In January, Public Health England said it did not recommend mixing COVID-19 vaccines from different suppliers.
The Russian vaccine uses a modified version of the common cold-causing adenovirus to carry genes for the coronavirus spike protein - so the body is primed to react if it encounters COVID-19.
It works in a similar way to the Oxford/AstraZeneca jab developed in the UK - but unlike that two-dose vaccine, the Russians used a slightly different adenovirus for the second booster shot.
The preliminary findings, published in The Lancet, are based on analysis of data from nearly 20,000 participants - three quarters of whom received the vaccine and one quarter had a placebo.
Russia approved the vaccine back in August, before the large-scale trial had begun, saying it was the first country to do so for a COVID-19 shot.
It named it Sputnik V, in homage to the world's first satellite, launched by the Soviet Union.
"The development of the Sputnik V vaccine has been criticised for unseemly haste, corner cutting, and an absence of transparency," said Ian Jones, professor at the University of Reading, and Polly Roy, professor at the London School of Hygiene and Tropical Medicine, who were not involved in the study.
"But the outcome reported here is clear and the scientific principle of vaccination is demonstrated," the scientists said in a comment shared by The Lancet.
"Another vaccine can now join the fight to reduce the incidence of COVID-19."
In the trial, participants were given two shots based on two different viral vectors and given 21 days apart.
The two vectors have been modified to express the SARS-CoV-2 spike protein.
The results, collated by the Gamaleya Institute in Moscow that developed and tested the vaccine, were in line with efficacy data reported at earlier stages of the trial, which has been running in the capital since September.
There were 2,144 volunteers aged over 60 in the trial and the shot was shown to be 91.8% effective when tested on this older group, with no serious side effects reported that could be associated with the vaccine, The Lancet said.