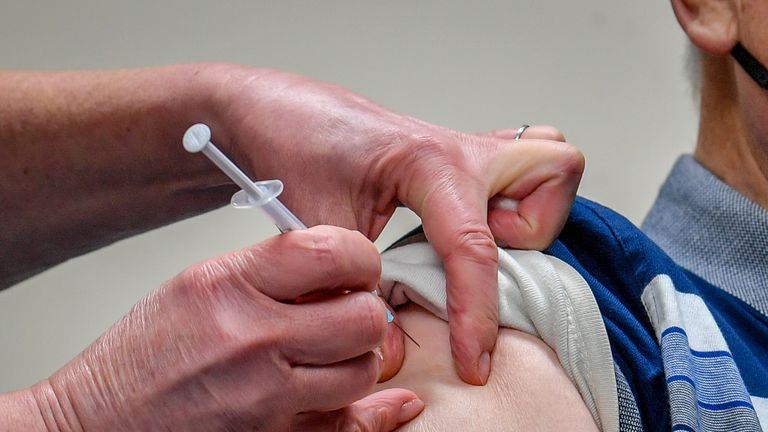
EU drug regulators have announced AstraZeneca's coronavirus vaccine can be given to adults in all age groups - the third jab cleared for use in the bloc.
The advice from the European Medicines Agency (EMA) comes after questions were raised in Germany over how effective the AstraZeneca jab, developed with Oxford University, is in protecting older people due to a lack of data.
And it is understood the German government will not be giving the AstraZeneca shot to the over-65s.
Live COVID updates from the UK and around the world
The EMA, which said the vaccine had an overall efficacy of around 60% in the trials, stated there were not yet enough results for people over the age of 55 to determine how well the vaccine would work for this group.
However it said protection was expected, given that an immune response is seen in this group - and the regulator ruled the vaccine can be given to older people.
Many countries on the continent have been struggling to vaccinate their populations as quickly as nations such as the UK.
There is currently a bitter wrangle between AstraZeneca and the EU over vaccine supply shortages in the bloc.
The European Commission has introduced measures to tighten rules on the exports of shots produced in the 27 EU countries.
The "vaccine export transparency mechanism'' will be used until the end of March to control vaccine shipments to non-EU countries - and to ensure any exporting company based in the EU first submits its plans to national authorities.
But the EU insisted the new measure is not an export ban - and the UK was not named among countries exempted from the plan.
The EU wants doses of the AstraZeneca vaccine to be sent from British plants to solve its jab shortage issues, after member states were forced to pause or delay rollout.
And if vaccines made within the bloc are prevented from being exported in future it could damage the UK's access to further supplies, particularly to the Belgian-made Pfizer jab.
In the AstraZeneca trials, only 12% of those who took part were over 55 and they were enrolled later, so there has not been enough time to collate the results.
On Thursday, a draft recommendation from Germany's vaccination advisory committee said the AstraZeneca vaccine should currently only be given to people aged 18-64.
UK regulators acknowledged the limited data for older people with regard to the AstraZeneca vaccine when it cleared the jab last month for people over 18.
But Public Health England has said details on the immune response for those 65 and over had been "reassuring".
A separate study testing the AstraZeneca vaccine in the US is still under way.
The vaccine is administered via two injections into the arm, the second 4-12 weeks after the first.
The EMA has already authorised the Pfizer and Moderna jabs for all adults.
Subscribe to the Daily podcast on Apple Podcasts, Google Podcasts, Spotify, Spreaker
Brussels hit out at AstraZeneca after the drug giant said it would reduce initial deliveries from 80 million doses to 31 million, blaming production problems.
EU chief Ursula von der Leyen has said the contract contained binding orders and the bloc has raised the threat of legal action to secure COVID-19 stocks.
AstraZeneca chief executive Pascal Soriot said the company was working 24/7 to increase capacity to the EU, adding it had millions of doses ready to start being sent to the bloc.
He also said glitches and delays in the manufacturing of coronavirus vaccines were inevitable given the speed at which they have been developed, but he would not comment on the EU dispute over supplies.