Event Highlights
- Vaccines to be Administered in 2 Dosages, Announces DCGI Somani
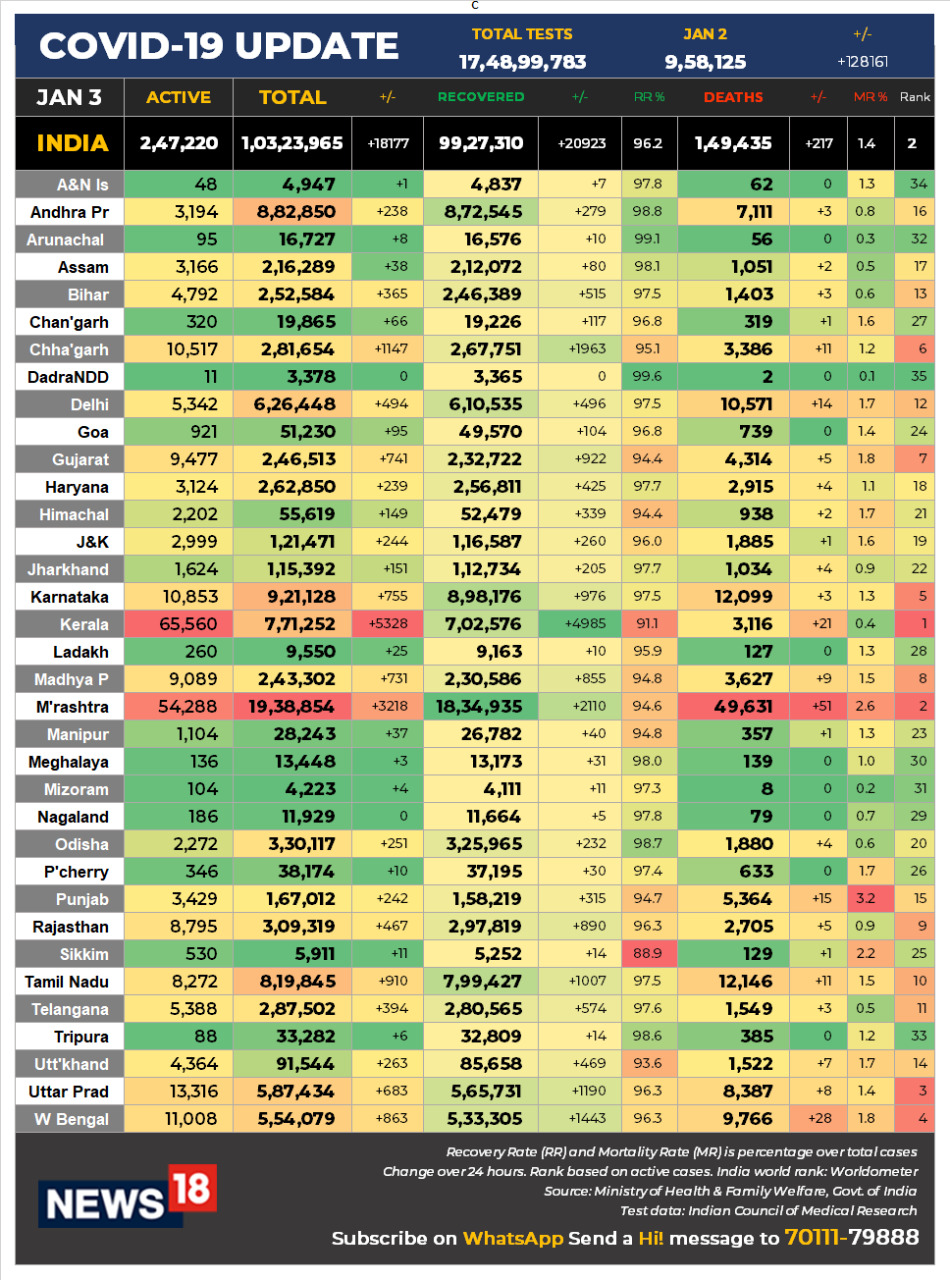
- India Reports 18,177 Fresh Cases, 217 Deaths
- Chennai Luxury Hotel Turns Covid Hotspot
- Health Minister Informs About Co-Win Platform
- Sourav Ganguly Tests Negative for Covid-19
- New Year’s Day Saw 160,606 New Cases, 2,051 Deaths in US
- Delhi Recorded 494 Fresh Cases, Lowest in Over 7 Months
- Akhilesh Dubs Country's Anti-Covid Vaccine as BJP's
The Subject Expert Committee had recommended both vaccines for approval in the last two days. The process for the final approval was expected to be a formality given the urgency for a vaccine in the country, but all eyes were on the dosage regime to be followed.
Bharat Biotech Recruits 23k Volunteers for Covid-19 Vaccine Trials | Bharat Biotech has successfully recruited 23,000 volunteers, and continued progress towards achieving the goal of 26,000 participants for Phase-3 clinical trial of its COVID-19 vaccine Covaxin across multiple sites in India, it said. The Phase III human clinical trials of Covaxin began mid-November, targeted to be done in 26,000 volunteers and it is the countrys first and only Phase III efficacy study for a COVID-19 vaccine, and the largest phase III efficacy trial ever conducted for any vaccine in India, a press release from the vaccine maker said on Saturday night.
READ | India First Nation to Successfully Isolate, Culture UK Coronavirus Variant, Says ICMR

India has successfully cultured the new coronavirus strain, which originated in the UK, the Indian Council of Medical Research (ICMR) said on Saturday.
Govt Should Lunch Covid-19 Inoculation Drive After Making Solid Arrangements: Akhilesh Yadav | In damage control mode after refusing to take “BJP’s vaccine”, Samajwadi Party chief Akhilesh Yadav on Sunday said the ruling party should not make a spectacle out of Covid-19 vaccination and should launch the inoculation drive only after making solid arrangements in advance. Taking to Twitter, Yadav also asked the government to announce the dates on which economically weaker sections of the population will be vaccinated.
कोरोना का टीकाकरण एक संवेदनशील प्रक्रिया है इसीलिए भाजपा सरकार इसे कोई सजावटी-दिखावटी इवेंट न समझे और अग्रिम पुख़्ता इंतज़ामों के बाद ही शुरू करे. ये लोगों के जीवन का विषय है अत: इसमें बाद में सुधार का ख़तरा नहीं उठाया जा सकता है.
— Akhilesh Yadav (@yadavakhilesh) January 3, 2021
गरीबों के टीकाकरण की निश्चित तारीख़ घोषित हो.
Today's Data Highlights
- 18.2k new cases, 20.9k new recoveries, 217 new deaths, 3k dip in active cases
- Active cases in India now below 2.5 lakh. India had similar number of active cases 6 months ago (on July 5, 2020)
- New cases below 20k for the second consecutive day. Below 25k for 14 days
- New deaths lowest in over 7 months (June 3)
- Kerala reports 5.3k new cases, Maharashtra 3.2k, Chhattisgarh 1.1k
- Maharashtra reports 51 new deaths, West Bengal 28, Kerala 21
India Reports 18,177 New Covid-19 Cases, 20,923 Recoveries, 217 Deaths | India reports 18,177 new COVID-19 cases, 20,923 recoveries, and 217 deaths in last 24 hours, as per Union Health Ministry. So far the total cases have reached 1,03,23,965, Active cases are at 2,47,220, total recoveries are- 99,27,310, while the death toll crossed 1,49,435
Chennai Luxury Hotel Turns Covid Hotspot, 85 Test Positive | About 85 people, including staff members of ITC Grand Chola in Guindy here,have tested positive for COVID-19 since December 15 last year, a senior official said here on Saturday. Of the total of 609 samples collected till date, 85 were found to be positive. Following this, the Greater Chennai Corporation has been instructed to carry out saturation testing of all the guests at the hotel, Health Secretary J Radhakrishnan said. ITC Grand Chola, in a release, said all events at the property have been conducted in adherence to the norms mandated by the authorities.
READ | India Likely to Approve 2-Dose Regimen for Covid-19 Vaccines, Four Weeks Apart, Says Report

India's drugs regulator is likely to approve administering two doses of the AstraZeneca-Oxford vaccine and another locally developed one by Bharat Biotech, each four weeks apart, a Reuters report said…
UK Hits Record 57,725 Daily Virus Cases | The UK has registered a record 57,725 daily coronavirus cases. Government figures show the UK has recorded five straight daily highs all above 50,000 and nearly double the levels of two weeks ago. Also, hospitals in Britain have started receiving batches of the coronavirus vaccine developed by Oxford University and AstraZeneca, approved by British regulators this week.
Russia Inoculates Over 800,000 People Against Covid-19, Issues Vaccination Certificates | More than 800,000 people in Russia have been inoculated so far against the new coronavirus and more than 1.5 million vaccine doses have been dispatched, Health Minister Mikhail Murashko said. Russia, which began rolling out its Sputnik V vaccine in early December, has the world's fourth higher number of COVID-19 cases and is putting high hopes on several vaccines it plans to produce.
READ | From August 15 Claim to Emergency Use Approval: The Road So Far to India's First Domestic Coronavirus Vaccine

India's drugs regulator on Saturday recommended for emergency use a locally developed coronavirus vaccine called COVAXIN, which is expected to be a backup to the AstraZeneca/Oxford shot.
Mexican Doctor Hospitalised After Receiving Covid-19 Vaccine | Mexican authorities said they are studying the case of a 32-year-old female doctor who was hospitalized after receiving the Pfizer-BioNTech COVID-19 vaccine. The doctor, whose name has not been released, was admitted to the intensive care unit of a public hospital in the northern state of Nuevo Leon after she experienced seizures, difficulty breathing, and a skin rash.
Dry Run for Covid-19 Vaccination Conducted in Chandigarh, Haryana's Panchkula District | Dry runs to check preparations for COVID-19 vaccination were held in Haryana's Panckula district and in the Union Territory of Chandigarh on Saturday. The dry run in Haryana was conducted in Panchkula at four sites with two sites each in urban and rural settings, Additional Chief Secretary (Health) Rajeev Arora said. "The sites in urban areas are in Sector-4 dispensary and Sector-8 dispensary, while in rural areas the sites are in Primary Health Centre-Kot and Primary Health Centre-Raipur Rani," he said in a statement. The platform of the existing universal immunisation programme of the state, with enhanced capacity, will be used for providing COVID-19 vaccine, said the senior Haryana official.
Health Minister Informs About Co-Win Platform to Facilitate Covid Inoculation
Co-WIN platform to facilitate:
— Dr Harsh Vardhan (@drharshvardhan) January 3, 2021
🔹Registration & verification of beneficiaries
🔸Scheduling inoculation
🔹SMS reminders for schedule & follow on dosage
🔸Reporting Adverse Event Following Immunisation
🔹e-Certificate post-vaccination pic.twitter.com/TP4ZHi8KPD
READ | Expert Panel Clears Bharat Biotech’s Covaxin Amid UK Virus Strain: What You Need to Know

An expert panel of India's central drug authority has recommended granting permission for restricted use of Bharat Biotech-developed indigenous COVID-19 vaccine Covaxin in emergency situation,…
Sourav Ganguly Tests Negative for Covid-19 | BCCI president and former India skipper Sourav Ganguly, who suffered a "mild" heart attack on Saturday and underwent a quick "primary angioplasty" for clearing a blocked coronary artery, tested negative for COVID-19, a doctor said. "Mr Ganguly tested negative for the COVID-19," he said, adding that the test was conducted before angioplasty was performed on him.
Vatican Likely to Start Administering Covid-19 Vaccinations in Mid-January | The Vatican says it expects to start administering COVID-19 vaccinations in mid-January. A statement on Saturday says vaccines, enough to cover the needs of the Holy See and of Vatican City State." The brief statement didn't say if 84-year-old Pope Francis would be getting the vaccine. But it specified priority would go to Vatican health and security workers, to the elderly and to the personnel most frequently in contact with the public.
US Braces for Post-holiday Covid Surge as Death Toll Nears 350,000 | The US is braced for a post-holiday coronavirus surge as its death toll nears 350,000, with thousands more predicted to die in the coming month and doctors warning they are at “breaking point”. New Year’s Day saw 160,606 new cases and 2,051 deaths, according to Johns Hopkins University, bringing the total caseload to 20.1 million and the death toll to 347,788.
Delhi Recorded 494 Fresh Cases, Lowest in Over 7 Months | Delhi recorded 494 fresh COVID-19 cases, the lowest in over seven months, and14 new fatalities on Saturday, even as the positivity rate stood at 0.73 per cent, authorities said. The infection tally in the city stands at over 6.26 lakh and the death toll rose to 10,561, they said. Delhi Health Minister Satyendar Jain tweeted that the positivity rate has been below one per cent for the past 11 days.
READ | India Grants Approval to Two Vaccines Against Covid-19, Sets Stage for Mass Rollout

The Indian government has granted an in-principle approval to two vaccines against Coronavirus, setting the stage for the roll out of the first phase of mass vaccination in the country.
Akhilesh Dubs Country's Anti-Covid Vaccine as BJP's, Draws Sharp Criticism | Samajwadi Party president Akhilesh Yadav termed the anti-Covid vaccine to be rolled out in the country a "vaccine of the BJP" and said he would not take the shot, prompting a sharp reaction not only from the ruling party but also from NC vice-president Omar Abdullah. While the ruling BJP accused Yadav of "insulting" doctors and scientists of the country, Abdullah said vaccines don't belong to any political party, but humanity.
England Health Officials Defend Contingency Plan to Mix Covid Vaccines | Officials have defended England’s vaccine regimen after details of a contingency plan to mix the two approved jabs in a small number of cases emerged. Public Health England’s Covid “green book” recommends that “it is reasonable to offer one dose of the locally available product to complete the schedule” if the same vaccine used for the first dose is not available. But it adds: “There is no evidence on the interchangeability of the Covid-19 vaccines although studies are under way," the Guardian reported.
Dry runs for Covid-19 inoculation being conducted across India.
Himachal Pradesh: Dry run for implementing the administration of #COVID19 vaccine was held at Deendyal Upadhyay Zonal Hospital in Shimla (2.1.2021) pic.twitter.com/K3KlrvNJhS
— ANI (@ANI) January 3, 2021
COVID-19 Vaccination Dry Run Conducted in Three Bihar Districts | A dry run for COVID vaccination was conducted in three districts of Bihar on Saturday. Altogether 75 personnel took part in the mock exercises that took place at three centres each in Patna, Jamui and West Champaran districts. Top officials of the department visited the primary health centres of Shastri Nagar and Phulwari Sharif in the state capital where the dry run was conducted.

Drugs Controller General of India Dr VG Somani
The regulator is likely to approve administering two doses of the AstraZeneca-Oxford vaccine and Covaxin, the locally developed one by Bharat Biotech, each four weeks apart, a Reuters report said on Sunday, quoting two sources. The Serum Institute of India, which is making the Oxford-AstraZeneca doses, had applied for a two full-dose regime about 28 days apart.
The other vaccine, known as COVAXIN, has been developed locally by Bharat Biotech and the government-run Indian Council of Medical Research. Citing sources, Reuters reported on Friday that the shot could be approved, though little is known about the results of its clinical trials. "Grant of permission for restricted use in emergency situation in public interest as an abundant precaution, in clinical trial mode, specially in the context of infection by mutant strains," the government cited the experts' recommendation for COVAXIN, referring to the new strain of the virus first detected in Britain.
For the AstraZeneca/Oxford vaccine, the approval was "subject to multiple regulatory conditionalities", it said, without giving details. Information and Broadcasting Minister Prakash Javadekar told reporters earlier that two other vaccines were waiting to be approved - Zydus Cadila's ZyCoV-D and Russia's Sputnik V that are both on trial in India. "India is perhaps the only country where four vaccines are getting ready," he said.
"One was approved yesterday for emergency use, Serum's COVISHIELD." he said, referring to the fact that the AstraZeneca/Oxford shot is being made locally by the Serum Institute of India (SII). The CDSCO is expected to announce the dosage and other details about the shot later. SII had applied for a two full-dose regime about 28 days apart.
The news of the sign-off on COVAXIN came after BJP lawmaker Subramanian Swamy complained on Twitter that a foreign-developed shot had been approved while a local one tested on thousands of people at home was "in the ditch". "We thank the citizens of India for showing confidence in an all Indian-made vaccine!" Bharat Biotech said in reply to Swamy's tweet, after its vaccine was recommended.
The AstraZeneca/Oxford vaccine, which was granted its first approval by Britain on Tuesday, is cheaper and easier to use than some rival shots, such as the one from Pfizer Inc - a major advantage in tackling a pandemic that has claimed more than 1.8 million lives worldwide. However, it has been plagued by uncertainty about its most effective dosage ever since data published in November showed a half dose followed by a full dose had a 90% success rate while two full shots were 62% effective. India's regulator has also received an emergency-use application for the COVID-19 vaccine made by Pfizer with Germany's BioNTech - the first shot to secure regulatory approval in the West.
India has reported more than 10.3 million COVID-19 cases and around 150,000 deaths, though its rate of infection has come down significantly from a mid-September peak. The country hopes to inoculate 300 million of its 1.35 billion people in the first six to eight months of this year. SII, the world's biggest producer of vaccines, has already stockpiled about 50 million doses of the AstraZeneca/Oxford shot, which will be sold to the government at about 250 rupees ($3.42) per dose and 1,000 rupees on the private market.


 LIVE NOW
LIVE NOW