Mesoblast: The Future Looks Bleak
I had high hopes for Mesoblast's revolutionary therapy after the ODAC thumbs up, and said so in my previous coverage.
However, the FDA's rejection of the NDA, and request for a new trial, makes Mesoblast's future look bleak in the USA.
I still believe they should be granted accelerated approval, but given the FDA's stance on the therapy, I would be surprised if they are given that.
Mesoblast (MESO) has had a terrible few months since August when I last covered it. In October, despite a 9-1 Advisory Committee vote for approval, its approval application for remestemcel-L got rejected by the FDA. In its CRL or Complete Response Letter, the FDA recommended that the Company “conduct at least 1 additional randomized, clinical study in adults and/or children to provide further evidence of the efficacy of remestemcel-L for SR-aGVHD.” This despite the company having conducted multiple phase 3 trials and an open label trial on the product candidate in pediatric GVHD showing 69% of Ryoncil patients had a complete or partial response to the treatment after 28 days. That last datapoint was from a single-arm study, Protocol MSB-GVHD001. Protocols 265 and 280, the two randomized trials, were not as successful.
Then on December 14 there was the news about the failure of Meso’s second asset, rexlemestrocel-L (REVASCOR®), to meet the primary endpoint of “reduction in recurrent non-fatal decompensated heart failure events” in the DREAM-HF Phase 3 randomized controlled trial in 537 patients with advanced chronic heart failure. Although rexlemestrocel-L was a known troublemaker and had previously had similar failures, this was a major blow to an already beaten down stock.
Finally, on December 17, Mesoblast had another trouble of Remestemcel-L in a trial in ventilator-dependent patients with moderate to severe acute respiratory distress syndrome (ARDS) due to COVID-19. Here, the Data Safety Monitoring Board (DSMB), after a third interim analysis on the trial’s first 180 patients, determined that the trial was not likely to meet the 30-day mortality reduction endpoint at the planned 300 patient enrolment. Although it was determined that during the initial stages of the pandemic, control mortality rates were exceedingly high and prior treatment regimens had reduced disease mortality in ventilated patients, the stock was unable to absorb the dual failures and fell sharply, going near $8, shedding almost 50% of its value.
So the question is, how is Mesoblast going to recover from all this?
It has been my opinion for a while now that Remestemcel-L in aGVHD is Mesoblast's core asset. REVASCOR, or even Remestemcel-L in other indications like ARDS in covid patients, are secondary issues that bolster the bull case if successful, but are not necessary for it. If the aGVHD indication is approved, it will be pioneering work from this stem cell developer from Australia. So, that is the core asset, and in order to argue for the retention of our investment thesis, we need to see how the company can work around the problem it is having with the FDA.
In the way the FDA rejected Mesoblast’s stem cell therapy after its years of trials and enthusiastic adcomm support, there have been questions surrounding this. People have asked whether the refusal is less to do with any inherent defect in the trial data and more to do with some sort of principled stand against stem cell therapy itself. According to the FDA’s own publications, “The only stem cell-based products that are FDA-approved for use in the United States consist of blood-forming stem cells (hematopoietic progenitor cells) derived from cord blood.” However, Mesoblast’s Ryoncil is already approved in Japan as Temcell, licensed to JCR Pharma. It is rare indeed that the FDA rejects a 9-1 vote from its own appointed advisory committee. I mean, what is the use of having expert advisors if you are not going to listen to their advice? These were also not just anybody else; these were top experts in the aGVHD area.
If that be the case, if this was a political, Republican party oriented decision, then a change in administration may actually be good for Mesoblast. The focus of political ire against stem cell research is hESC or human embryonic stem cell - see policy discussion here. However, that may not entirely bypass other sorts of stem cells, especially from companies outside the USA, and where a question can be raised about benefit and trial design. In its turn, the Democrats have supported stem cell research. Barack Obama lifted the George Bush era ban on hESC under certain conditions. The focus of the US FDA’s critique of stem cell therapy has not been adult stem cells, however, nor even induced pluripotent stem cells. In 2014, the FDA was studying MSCs itself. On the other hand, the FDA took action against Regenerative Sciences, LLC, which manufactured a product called Regenexx™, which consisted of autologous mesenchymal stem cells that were manipulated outside of the body and injected back into patients with orthopedic injuries.
In an old document from the FDA, I see the following ethical considerations discussed by them in stem cell research:
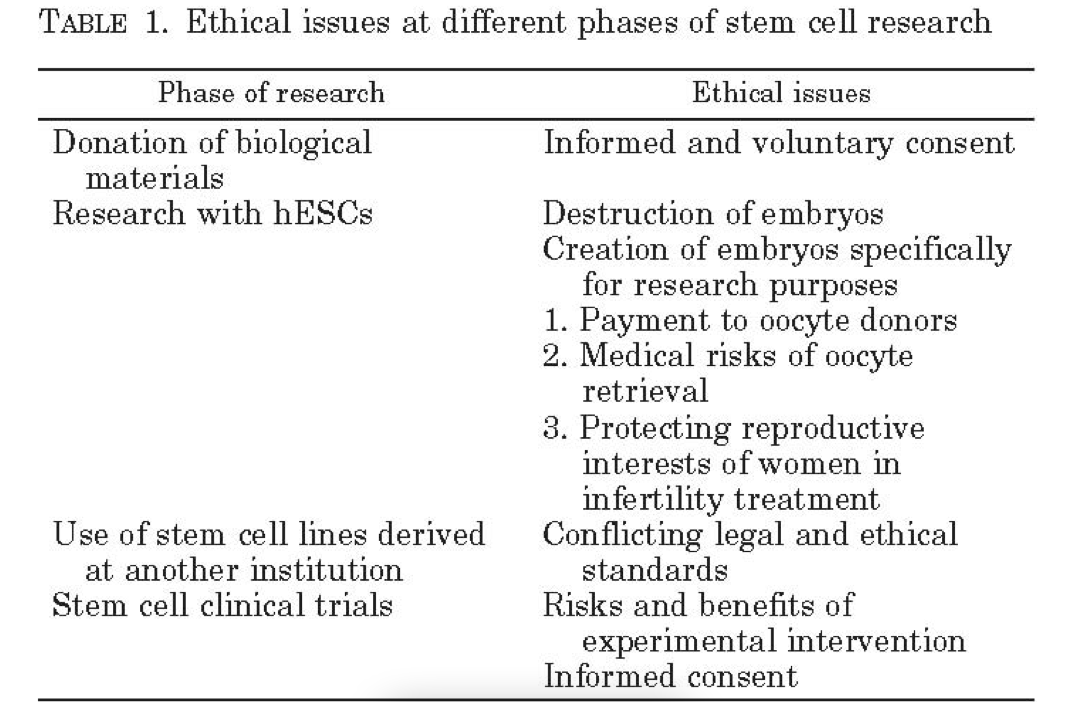
Now, Mesoblast uses Mesenchymal lineage cells that “are collected from the bone marrow of healthy adult donors and proprietary processes are utilized to expand them to a uniform, well characterized, and highly reproducible cell population.” Theoretically, the FDA should not have ethical problems with this.
In its briefing docs, the FDA said:
While remestemcel-L and other MSC-based investigational products have demonstrated apparent immunomodulatory effects in in vitro experiments, the ability of remestemcel-L to reduce inflammation as measured by inflammatory biomarkers in humans receiving the product has not been demonstrated. SR-aGVHD is thought to be an immune-mediated disorder but its etiology is complex and many cell types are likely to be involved in its pathogenesis. Therefore, any efficacy remestemcel-L might have in treating this disease is not sufficient to demonstrate the product’s mechanism of action.
And then proceeded to ignore all its own appointed experts who said otherwise.
The FDA also questioned Mesoblast’s manufacturing process. They said that "significant functional heterogeneity" exists between patients in other MSC-based therapies, and “the biotech's expansion of MSCs in culture through donor banks could dilute doses over time.”
What has irked investors is that these were questions that could well have been raised in meetings before the phase 3 trial was planned, apparently with the FDA’s blessing.
The covid-19 ARDS trial failure goes against the company’s efforts in aGVHD as well. What the FDA asked for in the CRL was at least 1 more randomized, controlled trial be conducted in adult and/or pediatric patients with SR-aGVHD to provide further evidence for the approval of this agent. According to some sources, “COVID-19 ARDS is an inflammatory disease with a similar profile to what has been observed in children with SR-GVHD.” Therefore, Ryoncil’s failure in the former population may have implications for its to-be-conducted phase 3 trial in the latter indication, despite already showing positive data there. The FDA has also questioned the 45% ORR protocol in the Meso trial as hypothetical.
In its briefing docs, the FDA called out three objections to Ryoncil: “Although the study reached the primary endpoint goal of a 28-day ORR at 69.1%, it is unclear whether this one single-arm trial provides evidence of clinical benefit in the treatment of SR-aGvHD in pediatric patients. Furthermore, it is unclear if the durability of response requires continued infusions of remestemcel-L. Finally, the relevance of the two previously conducted randomized, double-blind, placebo-controlled, multicenter studies that failed to meet their primary efficacy endpoints is uncertain.”
In the two topics of discussion, the FDA asked its experts:
Therefore, how are the results of one positive single-arm trial interpreted in a landscape of multiple negative clinical trials, including several randomized, controlled trials that failed to show a treatment effect of remestemcel-L?
Is an additional clinical trial in the SR-aGVHD population required for confirmation of the effectiveness of the product? What trial design trial would be required to provide evidence of effectiveness in this indication?
Now, although the experts answered in the positive to question 1 above, and in the negative to the 2nd question as to the need of a fresh trial, the FDA, diverging from its standard of usually agreeing with the experts at the adcomm, chose to disagree. That, in my opinion, does not bode well for Mesoblast. They have requested a Type A meeting around October end, which means that we should have known by now when this meeting is happening. We don’t. Mesoblast plans to ask for accelerated approval with patient access to Ryoncil while a phase 3 trial continues. That may not happen. I have done very well with my Mesoblast investment in the past couple years before the adcom, but the future looks bleak. Sadly, therefore, I plan to avoid this company until a lot of clarity is available on that question, and on whether a new 3-year trial can be actually completed by the company, given its finances.
About the author
Thanks for reading. At the Total Pharma Tracker, we offer the following:-

Our Android app and website features a set of tools for DIY investors, including a work-in-progress software where you can enter any ticker and get extensive curated research material.
For investors requiring hands-on support, our in-house experts go through our tools and find the best investible stocks, complete with buy/sell strategies and alerts.
Sign up now for our free trial, request access to our tools, and find out, at no cost to you, what we can do for you.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.

