FDA panel gives nod for emergency use approval for Pfizer vaccine
Washington, Dec 11: A panel of experts advising the US Food and Drug Administration has recommended the emergency approval for the the Pfizer-BioNTech Covid-19 vaccine.
The FDA's vaccine advisory panel, composed of independent scientific experts, infectious disease doctors and statisticians, voted 17 to 4, with one member, in favour of emergency authorisation for people aged 16 years or older, a report in The New York Times said.
Thanks to this the US may finally begin to slow the spread of the virus. On Wednesday, the US recorded more than 3,000 daily deaths.
 US experts convene to decide whether to OK Pfizer vaccine
US experts convene to decide whether to OK Pfizer vaccine
"I think the data was pretty compelling that the benefits greatly outweigh the risks. I wish there could have been slightly more enrollment of minorities in the trial. But I think the numbers were sufficient to make a decision," Dr. James Hildreth, a member of the FDA advisory committee said told CNN.
"We know at least in tens of thousands of people followed for two months, it doesn't have any serious, adverse events. ... I think we know enough now to say that this appears to be our way out of this awful, awful mess," said Dr Paul Offit, another member of the committee.
On December Britain began rolling out the Pfizer and BioNTech developed COVID-19 vaccine from today. Britain became the first western country to start vaccinating its general population.
The deployment of this vaccine marks a decisive turning point in the battle with the pandemic, said NHS chief executive Simon Stevens. NHS staff are proud to be leading the way as the first health service in the world to begin vaccination with this Covid jab, he also said.
Britain has ordered 40 million doses of the vaccine. Each person requires two doses and this would be enough to vaccinate 20 million people in the country of 67 million.
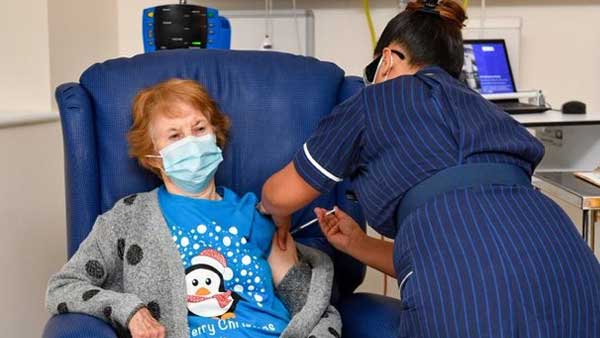 90 year old lady is first person in the world to receive Pfizer COVID-19 vaccine in UK
90 year old lady is first person in the world to receive Pfizer COVID-19 vaccine in UK
In the first week, around 800,000 doses are expected to be available, with care home residents and carers, those above 80 and some health service workers, the top priority get them.
United Kingdom became the first country in the world to approve the Pfizer/BioNTech coronavirus vaccine for widespread use.