Granules India shares gain on US FDA approval for generic of Cuprimine, Penicillamine capsules
Granules India announced that the US FDA has approved the ANDA filed by Granules Pharmaceuticals, Inc (GPI)., for Penicillamine Capsules USP, 250 mg, the company said in an exchange filing.
Dec 4, 2020 / 09:40 AM IST
Granules India: Company received approval from US FDA for generic of Cuprimine, Penicillamine capsules.
Granules India share price gained 2 percent at open on December 4 after the company received approval from US FDA for generic of Cuprimine, Penicillamine capsules.
Granules India Limited announced that the US Food & Drug Administration (US FDA) has approved the Abbreviated New Drug Application (ANDA) filed by Granules Pharmaceuticals, Inc (GPI)., a wholly owned foreign subsidiary of Granules India Limited, for Penicillamine Capsules USP, 250 mg, the company said in an exchange filing.
"It is bioequivalent to the reference listed drug product (RLD), Cuprimine of Bausch Health Americas, Inc. The product would be manufactured at Granules manufacturing facility in Chantilly, Virginia and is expected to be launched shortly," the company added.
Granules now has a total of 35 ANDA approvals from US FDA (33 Final approvals and 2 tentative approvals).
The stock was trading at Rs 426.95, up Rs 7.50, or 1.79 percent at 09:23 hours. It has touched an intraday high of Rs 430 and an intraday low of Rs 425.
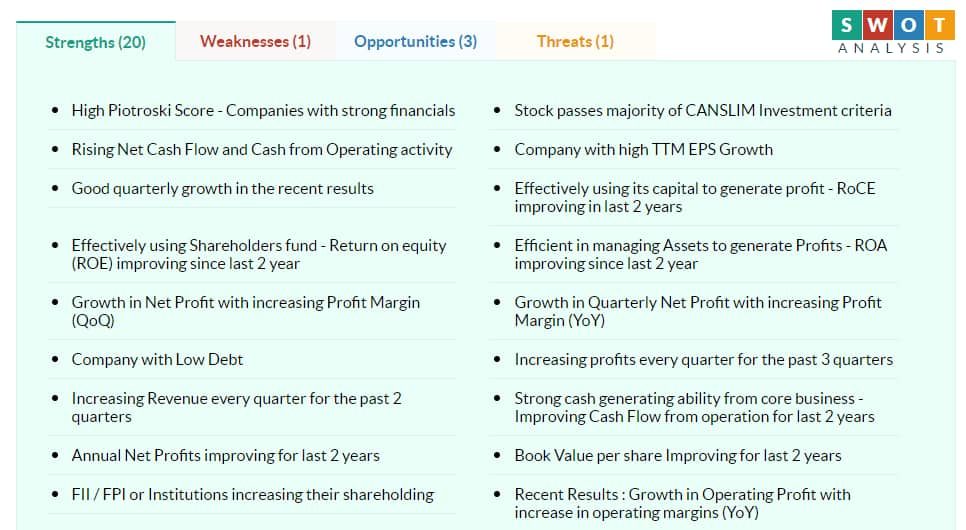
According to Moneycontrol SWOT Analysis powered by Trendlyne, the stock is showing strong momentum: price above short, medium and long term moving averages. The company's book value per share has been improving for last 2 years. FII / FPI or institutions are also increasing their shareholding.
Moneycontrol technical rating is very bullish with moving averages and technical indicators being bullish.
Disclaimer: The views and investment tips expressed by investment experts on moneycontrol.com are their own, and not that of the website or its management. Moneycontrol.com advises users to check with certified experts before taking any investment decisions.












_2020091018165303jzv.jpg)



























