The Real Catalysts Are Still Ahead For Gilead
GILD led the COVID-19 therapeutic race earlier this year with the emergency use of remdesivir approved in May.
GILD now has full approval and demand for remdesivir is still high.
Setbacks with filgotinib and spending on acquisitions could be weighing on the stock.
Competitor readouts represent a near-term catalyst for GILD in October/November.
Gilead Sciences (GILD) announced additional good news concerning remdesivir recently, but competitor readouts ahead mean enthusiasm could be short lived. This article takes a look at how competitors might spoil the party for GILD.
A catalyst has passed
October 22nd saw the approval of GILD's Veklury (remdesivir) for the treatment of COVID-19 requiring hospitalization (in patients 12 years and older, at least 40 kg in weight).
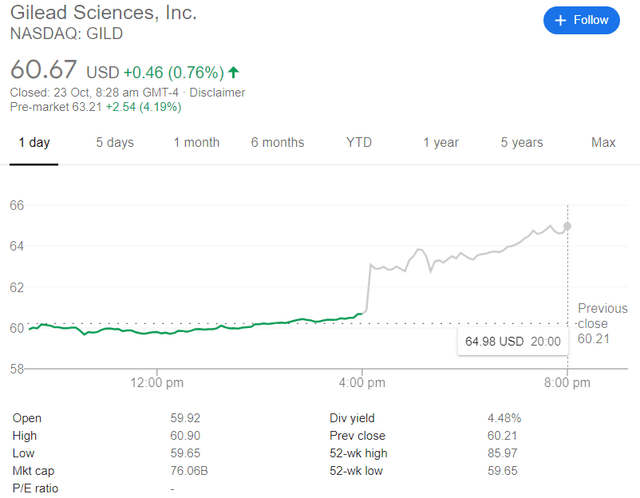
The reaction of the stock was positive afterhours on Thursday, with an initial 3% jump strengthening to a 7% run by 8 pm (Figure 1).
Figure 1: GILD trading on October 22, 2020. Source: Google.
Those following the name should look to see how well those gains hold up during Friday's session as GILD has been giving back its gains this year despite coming up with a drug for COVID-19. What has been weighing on the stock? There are some obvious answers. Firstly, there have been delays and issues with filgotinib, the company's anti-inflammatory drug being developed in collaboration with Galapagos NV (GLPG) in the US. Secondly there is possibly concern with the fact that GILD has been spending heavily. Seeking Alpha's Shock Exchange notes that at 15x 2024 revenues, the IMMU acquisition could be considered expensive and might limit GILD's ability to make other big acquisitions. There is another potential issue though.
GILD is exposed to readouts from COVID-19 vaccines
On October 22, Moderna (MRNA) announced it had completed enrolment (30,000 patients) of the phase 3 COVE study of mRNA-1273. Interim analyses in MRNA's trial are performed when the number of cases reaches 53 and then 106, with formal analysis of efficacy at 151 cases. Indeed MRNA's CEO, Stéphane Bancel recently suggested that positive interim results in November could see emergency use of the drug in December. That being said, Bancel also discussed the possibility that emergency use authorization could be delayed until 2021 for MRNA if the interim results take longer to acquire. Most recently a MRNA representative suggested the results of its interim analyses will be shared with the first such release coming around November 24/25.
With regards to Pfizer (PFE) and BioNTech (BNTX), we are perhaps even closer to a readout on efficacy. In an open letter on October 16, PFE CEO, Albert Bourla, noted that we might know if their COVID-19 vaccine works or not by the end of October. Assuming positive data then, PFE plans to apply for emergency use in the US soon after the third week of November, when there will be sufficient safety data for an application.
For GILD then, the idea would be that failure of these COVID-19 vaccines or delays only increases the relevance of remdesivir. The sooner the COVID-19 pandemic is under control, something a vaccine would help with greatly, the sooner orders for remdesivir would lessen. Of course remdesivir will still see some use, there are those who won't be vaccinated (by choice or for another reason) and there are those who will be vaccinated but might get COVID-19 anyway (unless these vaccines are 100% effective, which seems unlikely). Either way, remdesivir orders will continue for a while as stockpiling of the drug will occur once any excess demand is met. For example, on June 30 it was announced that the US had secured most of the next three months' supply of remdesivir. That stockpiling process could in fact be years away if remdesivir remains in short supply. Indeed in early October, reports arose of rationing of remdesivir in the UK, with shortages also reported in the Czech Republic and The Netherlands.
Conclusions
GILD's approval of remdesivir is a nice milestone for the company, but the real catalysts lie ahead with readouts from COVID-19 vaccines from PFE/BNTX and MRNA. Does remdesivir remain very much one of the key tools in the treatment arsenal to combat COVID-19 for quite a while? Or are we months away from a working vaccine and perhaps a couple of quarters away from widespread use of that vaccine? Such an event would lead to COVID-19 cases falling and remdesivir demand being met and eventually falling, allowing stockpiling to take place. That process could take years to play out, but would lead to models of remdesivir revenues being adjusted.
GILD could trade down if PFE and BNTX report positive results in the coming week or weeks. Alternatively, if a successful vaccine means the world is saved, then perhaps the whole market will rally, taking GILD with it. I see the former reaction as more likely. It seems the market doesn't like GILD so much of late and a simplistic thesis of "remdesivir needed less due to vaccine" would probably prevail. Since I think PFE/BNTX will succeed, I find myself bearish on GILD.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.