Coronavirus vaccine update: Oxford vaccine can still be ready by year-end, says AstraZeneca CEO
TIMESOFINDIA.COM | Last updated on - Sep 12, 2020, 10:30 ISTShare fbsharetwsharepinshareComments (0)
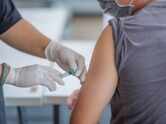
01/4Coronavirus vaccine update: Oxford vaccine can still be ready by year-end, says AstraZeneca CEO
)

After the Oxford-AstraZeneca vaccine, which was touted to be one of the most promising vaccine candidates for COVID-19, hit the roadblock this week, AstraZeneca CEO Pascal Soriot shared that the vaccine may still be available by the end of the year or early next year. The last stage human trials of the Oxford vaccine were halted after a volunteer developed serious neurological side-effects. It should be noted that the Phase II trials of the AstraZeneca-backed vaccine have been paused in India as well following a notice by the DGCI.
02/4Why were the trials of Oxford-AstraZeneca vaccine paused?
)

The trials of the COVID-19 vaccine, which is being jointly developed by the Oxford University and British-Swedish drug maker AstraZeneca were halted after a volunteer fell sick and developed adverse side-effects involving the spinal cord from the vaccine candidate. The pharmaceutical giant maintains that the pause in the trials was voluntary and is the usual course of action whenever there is a potentially unexplained illness in one of the trials.
03/4Will the Oxford vaccine get cancelled now?
)

While the pause in the trial does not mean that the Oxford vaccine candidate is out of the race of the potential vaccines for the novel coronavirus, the researchers will certainly take their time combing through the safety trial data of the vaccine and analyzing other incidents of side-effects. This would mean that the timeline of the completion of the critical last leg trials of the Oxford vaccine will only be pushed further. It should be noted that AstraZeneca had begun the last leg of human trials just month and a half earlier and was aiming to finish the human testing by November-end.
04/4“Oxford vaccine may still be available by the year-end, depending on regulatory approvals”
)

While the British drugmaker did not comment on the tentative timeline when the trial would resume, AstraZeneca Plc Chief Executive Officer, Pascal Soriot said that the Oxford coronavirus could still be made available by the end of 2020.
“I still think we are on track for having a set of data that we would submit before the end of the year for regulatory approval,” Pascal said at a media event.
He added that depending on “how fast the regulators move, they could still have a vaccine by the end of this year, early next year.”






























































![[Gym] Leg Training (voice-over with tips)
[Gym] Leg Training (voice-over with tips)](https://static.toiimg.com/thumb/77056786.cms?width=147&height=86)











closecomments
SIGN IN WITH
FacebookGoogleEmail