CHENNAI: The suspension of global clinical trials for Covishield, Covid-19 vaccine developed by Oxford University, will not affect phase-2 trials in Tamil Nadu, said officials.
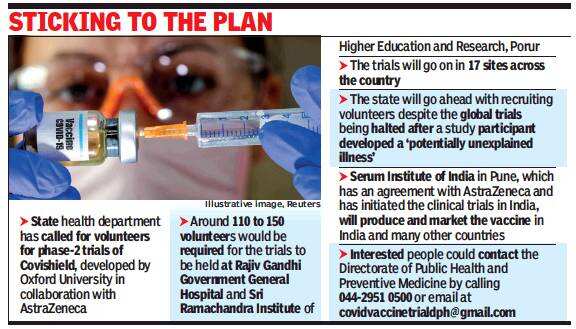
The state health department called for volunteers to register for screening through social media platforms, saying trials could even begin within a week if they get an adequate number of eligible candidates. “We are calling for volunteers to participate in trials. Once they are screened and if everything goes right, we will start may be within a week,” said Dr T S Selvavinayagam, director of public health and principal investigator of the project.
The suspension of global trials, he said, was only a small pause. “This is not stopping the trials. This is how vaccine trials have to go whenever there is an adverse event. It will be re-examined and monitored. Even if there is swelling in the area where the injection has been administered, we take that also as an adverse event and we will monitor.”
On Wednesday, reports emerged that AstraZeneca, collaborating with Oxford University, halted global phase-3 trials after a participant developed a ‘potentially unexplained illness’. A few other reports said the volunteer developed transverse myelitis, an inflammation in the spinal cord. Phase 2/3 trials are on in the US, UK, South Africa and Brazil.
In Tamil Nadu, trials will take place in Rajiv Gandhi Government General Hospital and Sri Ramachandra Institute of Higher Education and Research, Porur, vaccines for which reached Chennai on September 3. The two sites are part of the 17 sites in India chosen for trials.
Serum Institute of India (SII), which has an agreement with AstraZeneca and has clinical trials in the country, will produce and market the vaccine in India and many other countries. SII, in an online post, said, “We (Serum Institute of India) can’t comment on reports of AstraZeneca pausing trials in the UK, other than that they have been paused for review and shall restart soon. The Indian trials are continuing, and we have faced no issues at all.”
In July, results of phase 1/2 clinical trials in the UK published in The Lancet journal, said the vaccine triggered a response from T-cells — white blood cells that can attack infected cells — within 14 days of vaccination and an antibody response within 28 days. It also caused minor side effects like fever, chills and muscle pain, most of which reduced by using paracetamol. There were no serious adverse effects.
The state health department called for volunteers to register for screening through social media platforms, saying trials could even begin within a week if they get an adequate number of eligible candidates. “We are calling for volunteers to participate in trials. Once they are screened and if everything goes right, we will start may be within a week,” said Dr T S Selvavinayagam, director of public health and principal investigator of the project.

The suspension of global trials, he said, was only a small pause. “This is not stopping the trials. This is how vaccine trials have to go whenever there is an adverse event. It will be re-examined and monitored. Even if there is swelling in the area where the injection has been administered, we take that also as an adverse event and we will monitor.”
On Wednesday, reports emerged that AstraZeneca, collaborating with Oxford University, halted global phase-3 trials after a participant developed a ‘potentially unexplained illness’. A few other reports said the volunteer developed transverse myelitis, an inflammation in the spinal cord. Phase 2/3 trials are on in the US, UK, South Africa and Brazil.
In Tamil Nadu, trials will take place in Rajiv Gandhi Government General Hospital and Sri Ramachandra Institute of Higher Education and Research, Porur, vaccines for which reached Chennai on September 3. The two sites are part of the 17 sites in India chosen for trials.
Serum Institute of India (SII), which has an agreement with AstraZeneca and has clinical trials in the country, will produce and market the vaccine in India and many other countries. SII, in an online post, said, “We (Serum Institute of India) can’t comment on reports of AstraZeneca pausing trials in the UK, other than that they have been paused for review and shall restart soon. The Indian trials are continuing, and we have faced no issues at all.”
In July, results of phase 1/2 clinical trials in the UK published in The Lancet journal, said the vaccine triggered a response from T-cells — white blood cells that can attack infected cells — within 14 days of vaccination and an antibody response within 28 days. It also caused minor side effects like fever, chills and muscle pain, most of which reduced by using paracetamol. There were no serious adverse effects.

Coronavirus outbreak
Trending Topics
LATEST VIDEOS
City
 ‘Will expose you': Kangana Ranaut slams Uddhav Thackeray and Karan Johar 'gang' after BMC razes her office
‘Will expose you': Kangana Ranaut slams Uddhav Thackeray and Karan Johar 'gang' after BMC razes her office  Kangana vs Sena: Battle between demolishing house and demolishing ego
Kangana vs Sena: Battle between demolishing house and demolishing ego  Two Pakistani smugglers gunned down by BSF near Rajasthan border; pistols, drugs recovered from site
Two Pakistani smugglers gunned down by BSF near Rajasthan border; pistols, drugs recovered from site  Was BMC in a hurry to demolish Kangana Ranaut's home?
Was BMC in a hurry to demolish Kangana Ranaut's home?
More from TOI
Navbharat Times
Featured Today in Travel
Get the app





