The firm has got the nod for empagliflozin and linagliptin tablets. Empagliflozin is indicated to reduce the risk of cardiovascular death in adults with type 2 diabetes.
Alembic Pharmaceuticals share price gained 3 percent intraday on August 27 after the pharma company received a tentative nod for the US drug regulator for empagliflozin and linagliptin tablets.
The company has received US Food & Drug Administration (USFDA) tentative approval for empagliflozin and linagliptin Tablets, 10 mg/5 mg and 25 mg/5 mg. Alembic now has a total of 130 ANDA approvals (113 final approvals and 17 tentative approvals) from the FDA, the company said in an exchange filing.
Empagliflozin is indicated to reduce the risk of cardiovascular death in adults with type 2 diabetes mellitus and established cardiovascular disease. However, the effectiveness of empagliflozin and linagliptin tablets in reducing the risk of cardiovascular death in adults with type 2 diabetes mellitus and cardiovascular disease has not been established, the company said.
Empagliflozin and linagliptin tablets (10 mg/5 mg and 25 mg/5 mg) had an estimated market size of $244 million for twelve months ending June 2020, the company said, citing IQVIA data.
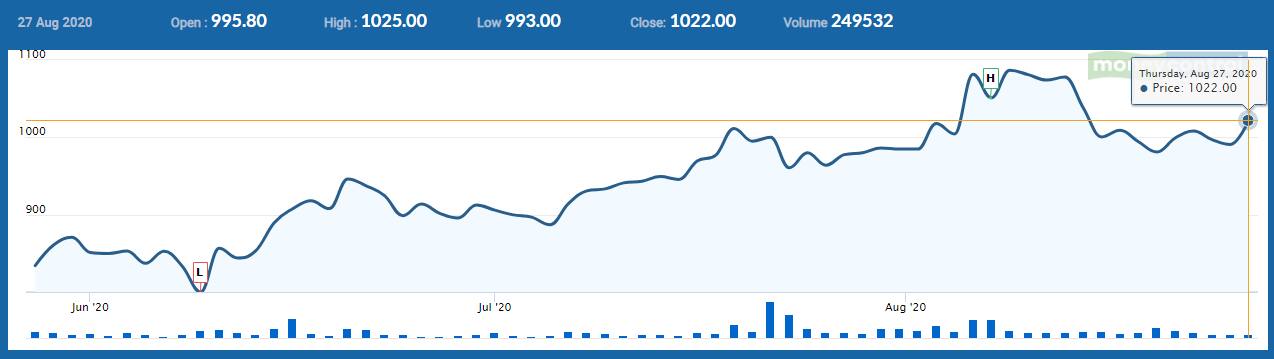
The stock, which has gained 57 percent in the past six months, was trading at Rs 1,022.40, up Rs 31.60, or 3.19 percent. It has touched an intraday high of Rs 1,024.45 and an intraday low of Rs 990.80.
The drug company on August 25 said its joint venture partner Aleor Dermaceuticals received final approval from the US regulator for Desonide lotion used to treat a variety of skin conditions. The approved Abbreviated New Drug Application (ANDA) is therapeutically equivalent to the reference listed drug product DesOwen Lotion, 0.05 percent, of Galderma Laboratories LP.
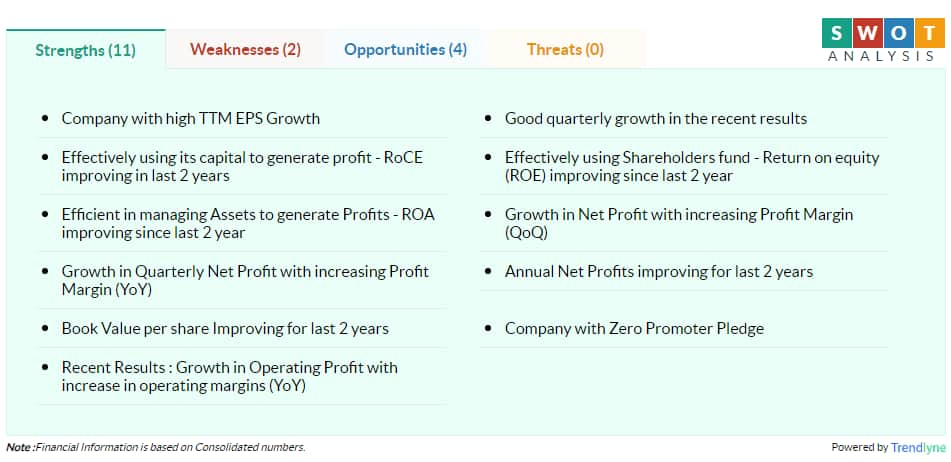
According to Moneycontrol SWOT Analysis powered by Trendlyne, the company has zero promoter pledge with book value per share improving for the past two years
Moneycontrol technical rating is bullish with moving averages and technical indicators being bullish.
Disclaimer: The views and investment tips expressed by experts on moneycontrol.com are their own and not those of the website or its management. Moneycontrol.com advises users to check with certified experts before taking any investment decisions.







