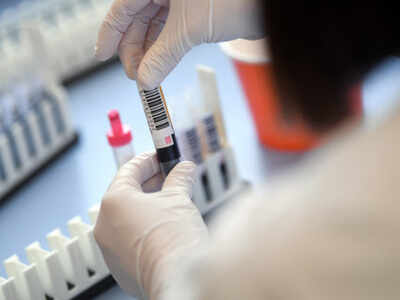
CHANDIGARH: One of the jobs of PGI during the clinical testing of Covid-19 vaccine candidate ‘Covidshield’ developed by Oxford University and pharmaceutical company Astra Zeneca will be to look into its immunogeneticity — antibody response. This will be part of the phase 2 and 3 trials for which the institute was selected among 17 others in the country. Not all the sites will evaluate immunogeneticity.
The antibody response is crucial as it determines the efficacy of the vaccine. Instead of the Elisa assay, CLIA (Chemiluminescence Immunoassays) shall be used to determine the antibody response once the volunteers are jabbed with Covidshield. “We have a fully automated CLIA technique which we had used for convalescent plasma therapy. It is more specific and sensitive than Elisa,” said Prof Mini P Singh, virologist at PGI and associated with the Oxford vaccine trials.
She said, “Initially, we will test for antibodies in PGI. But for further study of the neutralising antibody evaluation, samples shall be sent to NIV, Pune, which has the BS3 level facilities to deal with the live viruses."
The trials shall start by the third week of this month with around 250 volunteers. The crucial component will be immunogeneticity. “For this, we shall test the samples after a month of the shot. It takes around 4-6 weeks for the antibodies to respond. To understand if these antibodies are persistent, we will again evaluate after three months,” said Prof Singh.
An antibody test is designed to detect antibodies produced in response to a virus. The test relies on testing the blood to check for past infection. Antibodies are proteins that help fight off infections.
The antibody response is crucial as it determines the efficacy of the vaccine. Instead of the Elisa assay, CLIA (Chemiluminescence Immunoassays) shall be used to determine the antibody response once the volunteers are jabbed with Covidshield. “We have a fully automated CLIA technique which we had used for convalescent plasma therapy. It is more specific and sensitive than Elisa,” said Prof Mini P Singh, virologist at PGI and associated with the Oxford vaccine trials.
She said, “Initially, we will test for antibodies in PGI. But for further study of the neutralising antibody evaluation, samples shall be sent to NIV, Pune, which has the BS3 level facilities to deal with the live viruses."
The trials shall start by the third week of this month with around 250 volunteers. The crucial component will be immunogeneticity. “For this, we shall test the samples after a month of the shot. It takes around 4-6 weeks for the antibodies to respond. To understand if these antibodies are persistent, we will again evaluate after three months,” said Prof Singh.
An antibody test is designed to detect antibodies produced in response to a virus. The test relies on testing the blood to check for past infection. Antibodies are proteins that help fight off infections.

Coronavirus outbreak
Trending Topics
LATEST VIDEOS
City
 Rajasthan: 11 Pakistani refugees found dead in Jodhpur
Rajasthan: 11 Pakistani refugees found dead in Jodhpur  Kozhikode air crash: Deceased co-pilot Akhilesh Kumar survived by pregnant wife
Kozhikode air crash: Deceased co-pilot Akhilesh Kumar survived by pregnant wife  Kozhikode air mishap: Findings of the investigation will be made public, says Civil aviation minister Hardeep Singh Puri
Kozhikode air mishap: Findings of the investigation will be made public, says Civil aviation minister Hardeep Singh Puri  Delhi: No Covid effect? Fancy numbers find takers
Delhi: No Covid effect? Fancy numbers find takers
More from TOI
Navbharat Times
Featured Today in Travel
Quick Links
Kerala Coronavirus Helpline NumberHaryana Coronavirus Helpline NumberUP Coronavirus Helpline NumberBareilly NewsBhopal NewsCoronavirus in DelhiCoronavirus in HyderabadCoronavirus in IndiaCoronavirus symptomsCoronavirusRajasthan Coronavirus Helpline NumberAditya ThackerayShiv SenaFire in MumbaiAP Coronavirus Helpline NumberArvind KejriwalJammu Kashmir Coronavirus Helpline NumberSrinagar encounter
Get the app





