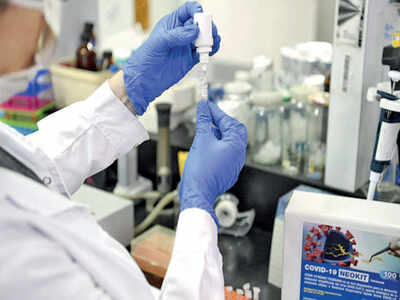
PANAJI: Redkar Hospital in North Goa has collected samples of 30 volunteers for human trials of India’s first potential Covid-19 vaccine. The hospital, which is among the 12 listed for the trials, expects to have within three days a total of 130 healthy volunteers in the age group 15 to 55. Thursday was day two for registration for the trial.
“Human trials of #Covaxin, an indigenously developed vaccine for #COVID19 has begun at Redkar Hospital in Goa. This is a testimony of India's immense potential in healthcare innovation. My best wishes to the entire team working on #Covaxin. #IndiaFightsCOVID19,” chief minister Pramod Sawant tweeted.
The samples will be sent to three laboratories — National Institute of Virology (NIV), Dr Dangs Lab, New Delhi, and Bharat Biotech, Hyderabad — for testing and only after the results are received eligible volunteers will be called for trials, said Dr Dhananjay Lad, director at CROM Clinical and Medical Tourism Pvt Ltd, which has been selected for conducting trials at Redkar Hospital.
“We haven’t had a problem in getting volunteers, but we want educated people who understand the whole concept of vaccine trial,” he said.
“After the vaccine is injected, which will be within a week of receiving laboratory reports of the samples, volunteers will be monitored for two hours and will be asked to go home after that,” Lad said.
Volunteers will be called 14 days after the vaccine is injected to test the level of antibodies in their system, he said.
Bhavesh Zambaulikar, 41, who is the president of the Bharatiya Janata Party yuva morcha, is one of the few first volunteers. He said he had been pursuing the development of the vaccine and a few months ago had also written to Bharat Biotech offering to be a volunteer.
“There was no response but later I learnt that the trial could take place in Nashik. I was willing to go there as well. But last month, I learnt that Redkar Hospital was one of the hospitals selected for conducting human trials for the vaccine. I immediately contacted the hospital,” he said.
Zambaulikar signed the documents on Tuesday giving his nod in writing and also convinced some of his friends to participate in the trial. “They want educated people for the trial. It takes about an hour or more to explain how the trial will unfold, starting with an explanation of what a virus is. A doctor on job will have to spend more time explaining if the volunteer is uneducated,” he said.
“Human trials of #Covaxin, an indigenously developed vaccine for #COVID19 has begun at Redkar Hospital in Goa. This is a testimony of India's immense potential in healthcare innovation. My best wishes to the entire team working on #Covaxin. #IndiaFightsCOVID19,” chief minister Pramod Sawant tweeted.
The samples will be sent to three laboratories — National Institute of Virology (NIV), Dr Dangs Lab, New Delhi, and Bharat Biotech, Hyderabad — for testing and only after the results are received eligible volunteers will be called for trials, said Dr Dhananjay Lad, director at CROM Clinical and Medical Tourism Pvt Ltd, which has been selected for conducting trials at Redkar Hospital.
“We haven’t had a problem in getting volunteers, but we want educated people who understand the whole concept of vaccine trial,” he said.
“After the vaccine is injected, which will be within a week of receiving laboratory reports of the samples, volunteers will be monitored for two hours and will be asked to go home after that,” Lad said.
Volunteers will be called 14 days after the vaccine is injected to test the level of antibodies in their system, he said.
Bhavesh Zambaulikar, 41, who is the president of the Bharatiya Janata Party yuva morcha, is one of the few first volunteers. He said he had been pursuing the development of the vaccine and a few months ago had also written to Bharat Biotech offering to be a volunteer.
“There was no response but later I learnt that the trial could take place in Nashik. I was willing to go there as well. But last month, I learnt that Redkar Hospital was one of the hospitals selected for conducting human trials for the vaccine. I immediately contacted the hospital,” he said.
Zambaulikar signed the documents on Tuesday giving his nod in writing and also convinced some of his friends to participate in the trial. “They want educated people for the trial. It takes about an hour or more to explain how the trial will unfold, starting with an explanation of what a virus is. A doctor on job will have to spend more time explaining if the volunteer is uneducated,” he said.
Quick Links
Kerala Coronavirus Helpline NumberHaryana Coronavirus Helpline NumberUP Coronavirus Helpline NumberBareilly NewsBhopal NewsCoronavirus in DelhiCoronavirus in HyderabadCoronavirus in IndiaCoronavirus symptomsCoronavirusRajasthan Coronavirus Helpline NumberAditya ThackerayShiv SenaFire in MumbaiAP Coronavirus Helpline NumberArvind KejriwalJammu Kashmir Coronavirus Helpline NumberSrinagar encounter
Get the app








