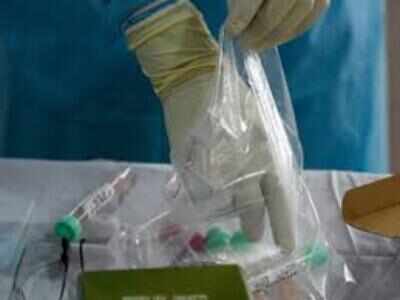
CHENNAI: Seniors citizens above 60 years of age are being given anti-tuberculosis shots, that are normally given to children as part of a national trial to study the vaccine's effect in preventing Covid-19, health minister C Vijaya Baskar said.
“We do not have any drug or vaccine to cure the disease yet. TN is part of the study by ICMR. The Bacille Calmette-Guerin (BCG) vaccines are expected to help people between 65 and 90 years,” he said.
According to Dr Manoj V Murhekar from the National Institute for Research in Tuberculosis (NIRT), six high incidence states including Tamil Nadu, Gujarat, Rajasthan, Madhya Pradesh, Maharashtra and Delhi will be part of the study. In Tamil Nadu, the elderly population from Chennai and Tiruvallur will receive the vaccine.
Experts suggest that this vaccine, which prevents infant death, helps the immune system recognize and respond to a variety of infections, including viruses.
“It is not clear if BCG will block Covid-19, but clinical trials are being done across the globe. We are now initiating the study in the most vulnerable population where fatalities are high,” he said. Certain studies done among adults have revealed that BCG vaccinations reduced the incidence of acute upper respiratory tract infections.
The team has started administering the live-attenuated vaccine (LAV), which comprises live but weakened TB virus, to thousands of senior citizens as part of the trial since Monday, said deputy director Dr Padmapriyadarsini C, the principal investigator of this study. Doctors and health workers are expected to vaccinate at least 1,200 senior citizens across the country by July end.
“We are planning to conduct camps to screen the elderly. BCG vaccinations cannot be administered to anyone who has a compromised immune system. We have to choose candidates carefully,” added Dr Padmapriyadarsini C. For instance, people with immune-compromised conditions such as cancer or HIV/AIDS, those on dialysis, or the ones who have undergone organ transplant will not be eligible to take the vaccine. Those undergoing treatment for TB or were under treatment for the disease in the last six months will also not be eligible.
Patients given the vaccine would be followed up for at least six months as part of the study, she said.
For details email: directornirt@nirt.res.in or call: 91-44-2836 9503/00
“We do not have any drug or vaccine to cure the disease yet. TN is part of the study by ICMR. The Bacille Calmette-Guerin (BCG) vaccines are expected to help people between 65 and 90 years,” he said.
According to Dr Manoj V Murhekar from the National Institute for Research in Tuberculosis (NIRT), six high incidence states including Tamil Nadu, Gujarat, Rajasthan, Madhya Pradesh, Maharashtra and Delhi will be part of the study. In Tamil Nadu, the elderly population from Chennai and Tiruvallur will receive the vaccine.
Experts suggest that this vaccine, which prevents infant death, helps the immune system recognize and respond to a variety of infections, including viruses.
“It is not clear if BCG will block Covid-19, but clinical trials are being done across the globe. We are now initiating the study in the most vulnerable population where fatalities are high,” he said. Certain studies done among adults have revealed that BCG vaccinations reduced the incidence of acute upper respiratory tract infections.
The team has started administering the live-attenuated vaccine (LAV), which comprises live but weakened TB virus, to thousands of senior citizens as part of the trial since Monday, said deputy director Dr Padmapriyadarsini C, the principal investigator of this study. Doctors and health workers are expected to vaccinate at least 1,200 senior citizens across the country by July end.
“We are planning to conduct camps to screen the elderly. BCG vaccinations cannot be administered to anyone who has a compromised immune system. We have to choose candidates carefully,” added Dr Padmapriyadarsini C. For instance, people with immune-compromised conditions such as cancer or HIV/AIDS, those on dialysis, or the ones who have undergone organ transplant will not be eligible to take the vaccine. Those undergoing treatment for TB or were under treatment for the disease in the last six months will also not be eligible.
Patients given the vaccine would be followed up for at least six months as part of the study, she said.
For details email: directornirt@nirt.res.in or call: 91-44-2836 9503/00

Coronavirus outbreak
Trending Topics
LATEST VIDEOS
City
 Watch: 101-year-old Mumbai man recovers from Covid-19, hospital staff celebrates his birthday
Watch: 101-year-old Mumbai man recovers from Covid-19, hospital staff celebrates his birthday  On cam: Drunk man falls into 100-feet-deep dilapidated well, rescued in Andhra Pradesh
On cam: Drunk man falls into 100-feet-deep dilapidated well, rescued in Andhra Pradesh  Love for photography: Man builds 3-storey camera-shaped house in Karnataka’s Belagavi
Love for photography: Man builds 3-storey camera-shaped house in Karnataka’s Belagavi  Kochi school teacher uses Augmented Reality Technology for conducting Lower Primary online classes
Kochi school teacher uses Augmented Reality Technology for conducting Lower Primary online classes
More from TOI
Navbharat Times
Featured Today in Travel
Get the app





