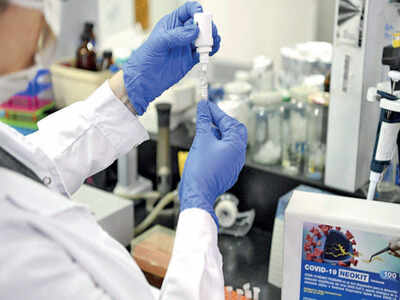
BENGALURU: Three members of the National Biomedical Resource Indigenisation Consortium (N-BRIC), which is headquartered in Bengaluru, are ready to take three innovations related to coronavirus testing to the production stage.
The consortium was set up by the department of biotechnology on May 14 to drive innovation in India. Led by the Centre for Cellular and Molecular Platforms (CCAMP), it’s a public-private effort focused on developing reagents, diagnostics, vaccines and therapeutics for Covid-19 and other conditions.
Bengaluru-based Syngene International, a member of the consortium, has developed an ELISA antibody testing kit and tied up with bioscience firm HiMedia Laboratories for manufacturing and distribution of the product. While RT-PCR kits are the gold standard in Covid-19 diagnostics, rapid antibody tests are a globally recognised method for quick surveillance and community risk management.
Most antibody kits are imported. But global supplychain disruptions and doubts about the quality and efficacy of imported kits have prompted India to look for home-grown solutions.
“The kits (developed locally) will be sent to the National Institute of Virology in Pune for validation. Once approved, they will be a major enabling step towards Aatma Nirbhar Bharat in terms of enhancing testing capabilities,” said an N-BRIC spokesperson, referring to Prime Minister Narendra Modi’s call for self-reliance. “It (innovation) also comes at an extremely opportune time, when the ICMR has widened the testing protocol to survey for community exposure, especially among vulnerable groups, through rapid ELISA tests.”
C-CAMP chief executive officer Taslimarif Saiyed also said the development was a positive sign for local manufacturing. “The Syngene-HiMedia collaboration is an exciting development with potential far-reaching impact on our testing capacity,” he said. Saiyed is convener of the N-BRIC’s governing council Another Bengaluru firm, Richcore LifeSciences, has produced two key enzymes needed for RT-PCR kits. This could remove a major bottleneck in India’s efforts to massproduce diagnostic tools.
“Richcore, with help of scientists from IISER Chandigarh/Pune and IISC, has optimised and produced two enzymes, taq polymerase and reverse transcriptase, needed for RT-PCR kits. These enzymes are currently not produced at scale in India and require cGMP [current Good Manufacturing Practices] certification,” N-BRIC said. R Subramani, chief managing director of Richcore, said the firm was sending enzyme samples to test kit manufacturers to confirm stability and consistency. Once approved, the firm will mass-produce cGMP-certified enzymes for millions of kits in a few weeks’ time.
“I am pleased to see Richcore respond so rapidly to develop these key components for RT-PCR tests, which is a reflection of their expertise. I am confident that we will have 100 per cent made-in-India Covid-19 testing kits in the imminent future,” said Biocon chief Kiran Mazumdar Shaw, who is the chair of N-BRIC’s governing council.
Genei Laboratories, a Bengaluru-based biotech company, is also gearing up for large-scale production of kits developed by IIT-Delhi. The work will be done at an assembly and manufacturing facility specially created for Covid-19 diagnostic units at Andhra Pradesh MedTech Zone, Visakhapatnam.
“The testing method, developed by researchers at IIT-Delhi’s Kusuma School of Biological Sciences, has received ICMR’s validation for a sensitivity and specificity of 100 per cent,” Genei Laboratories said.
The consortium was set up by the department of biotechnology on May 14 to drive innovation in India. Led by the Centre for Cellular and Molecular Platforms (CCAMP), it’s a public-private effort focused on developing reagents, diagnostics, vaccines and therapeutics for Covid-19 and other conditions.
Bengaluru-based Syngene International, a member of the consortium, has developed an ELISA antibody testing kit and tied up with bioscience firm HiMedia Laboratories for manufacturing and distribution of the product. While RT-PCR kits are the gold standard in Covid-19 diagnostics, rapid antibody tests are a globally recognised method for quick surveillance and community risk management.
Most antibody kits are imported. But global supplychain disruptions and doubts about the quality and efficacy of imported kits have prompted India to look for home-grown solutions.
“The kits (developed locally) will be sent to the National Institute of Virology in Pune for validation. Once approved, they will be a major enabling step towards Aatma Nirbhar Bharat in terms of enhancing testing capabilities,” said an N-BRIC spokesperson, referring to Prime Minister Narendra Modi’s call for self-reliance. “It (innovation) also comes at an extremely opportune time, when the ICMR has widened the testing protocol to survey for community exposure, especially among vulnerable groups, through rapid ELISA tests.”
C-CAMP chief executive officer Taslimarif Saiyed also said the development was a positive sign for local manufacturing. “The Syngene-HiMedia collaboration is an exciting development with potential far-reaching impact on our testing capacity,” he said. Saiyed is convener of the N-BRIC’s governing council Another Bengaluru firm, Richcore LifeSciences, has produced two key enzymes needed for RT-PCR kits. This could remove a major bottleneck in India’s efforts to massproduce diagnostic tools.
“Richcore, with help of scientists from IISER Chandigarh/Pune and IISC, has optimised and produced two enzymes, taq polymerase and reverse transcriptase, needed for RT-PCR kits. These enzymes are currently not produced at scale in India and require cGMP [current Good Manufacturing Practices] certification,” N-BRIC said. R Subramani, chief managing director of Richcore, said the firm was sending enzyme samples to test kit manufacturers to confirm stability and consistency. Once approved, the firm will mass-produce cGMP-certified enzymes for millions of kits in a few weeks’ time.
“I am pleased to see Richcore respond so rapidly to develop these key components for RT-PCR tests, which is a reflection of their expertise. I am confident that we will have 100 per cent made-in-India Covid-19 testing kits in the imminent future,” said Biocon chief Kiran Mazumdar Shaw, who is the chair of N-BRIC’s governing council.
Genei Laboratories, a Bengaluru-based biotech company, is also gearing up for large-scale production of kits developed by IIT-Delhi. The work will be done at an assembly and manufacturing facility specially created for Covid-19 diagnostic units at Andhra Pradesh MedTech Zone, Visakhapatnam.
“The testing method, developed by researchers at IIT-Delhi’s Kusuma School of Biological Sciences, has received ICMR’s validation for a sensitivity and specificity of 100 per cent,” Genei Laboratories said.

Coronavirus outbreak
Trending Topics
LATEST VIDEOS
City
 BJP leader Sonali Phogat slaps market committee secretary in Haryana’s Hisar, video goes viral
BJP leader Sonali Phogat slaps market committee secretary in Haryana’s Hisar, video goes viral  King cobra found at a house in Odisha, later rescued
King cobra found at a house in Odisha, later rescued  Deva Snana Purnima: Lord Jagannath and divine siblings bathed, dressed in elephant costumes in Odisha's Rourkela
Deva Snana Purnima: Lord Jagannath and divine siblings bathed, dressed in elephant costumes in Odisha's Rourkela  1,100 villages of Haryana to get 1.15 crore fruit-bearing, shady trees, says Haryana minister
1,100 villages of Haryana to get 1.15 crore fruit-bearing, shady trees, says Haryana minister
More from TOI
Navbharat Times
Featured Today in Travel
Get the app