ABOUT AUTHORS
D.Visagaperumal*, Justin Ebuka Ezekwem, Hemaprasad Munji, Vineeth Chandy
Department of Pharmaceutical Chemistry,
T. John College of Pharmacy, Bangalore, Karnataka, India
*vishak_dr@yahoo.co.in
ABSTRACT
The review article is focused on studies of Isatin-based Schciff Bases and their biological and pharmacological activities. Isatin-based Schiff base are generally synthesized by condensation of the keto group of Isatin with different aromatic primary amines carrying imine or azomethine (–C=N–) functional group. Isatin Schciff Base possesses numerous biological properties like antitumor, antimicrobial, anti-inflammatory, anticonvulsant, antiviral, anti HIV, antioxidant, CNS depressant activities
INTRODUCTION
Isatin or 1H-indole-2, 3-dione (1) is an indole derivative. The compound was first obtained by Erdman and Laurent in 1841 (Otto Linne Erdmann, 1840) . Isatin is an important class of heterocyclic compounds. Recently, heterocyclic compounds analogues and their derivatives have attracted strong interest in medicinal chemistry due to their biological and pharmacological properties. (Manju P et. al., 2011). The small and simple isatin nucleus possesses numerous biological properties like antimicrobial (Singh UK et. al., 2010), anti HIV (Pandeya SN et. al., 1999), antitubercular (Ozlen G et. al., 2008), antitumor (Hoyun L et. al., 2009), anti-inflammatory (Gummadi SB et. al., 2010), antioxidant (Prakash CR et. al., 2011), antiviral (Shibinskya MO et. al., 2010), anticonvulsant (Prince PS et. al., 2009) and CNS depressant activities (Zapata-Sudo G et. al., 1986).
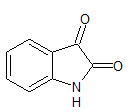
1
In nature, isatin is found in plants of the genus Isatis12, in Calanthe discolor (Yoshikawa M et. al., 1998), in Couroupita guianensis Aubl (Bergman J et. al., 1985), has also been found as a component of the secretion from the parotid gland of Bufo frogs (Wei L et. al., 1982) and in humans as it is a metabolic derivative of adrenaline (Ischia M et. al., 1988), ( Palumbo A et. al., 1989). Substituted isatins are also found in plants, for example the melosatin alkaloids (methox phenylpentyl isatins) obtained from the Caribbean tumorigenic plant Melochia tomentosa (Kapadia GJ et. al., 1980), ( Kapadia GJ et. al., 1977) as well as from fungi: 6-(3’-methylbuten-2’-yl)isatin was isolated from Streptomyces albus (Grafe U and Radics L, 1986) and 5-(3’-methylbuten-2’-yl)isatin from Chaetomium globosum (Breinholt J et. al., 1996). Isatin is one of the most promising new classes of heterocyclic molecules having many interesting activity profiles and well-tolerated in human (Yan Y et. al., 1992), (Joaquim FM et. al., 2001)
Synthesis of Isatin
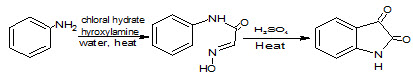
2
It may be prepared from cyclizing the condensation product of chloral hydrate, aniline and hydroxylamine in sulfuric acid (Marvel CS and Hiers GS, 1925), (Sandmeyer T, 1919). This reaction is called the Sandmeyer isonitrosoacetanilide isatin synthesis (2) and discovered by Traugott Sandmeyer in 1919. The method applies well to anilines with electron withdrawing substituents, such as fluoroaniline (Alam M et. al., 1989)
Schiff Bases
Schiff bases are the compounds carrying imine or azomethine (–C=N–) functional group. These are the condensation products of primary amines with carbonyl compounds and were first reported by Hugo Schiff (Schiff H, 1864), (Dhar DN and Taploo CL, 1982), (Sathe BS et. al., 2011) Schiff bases form an important class of the most widely used organic compounds and has a wide variety of applications in many fields including analytical, biological, and inorganic chemistry. Schiff bases have gained importance in medicinal and pharmaceutical fields due to a broad spectrum of biological activities like anti-inflammatory (Sondhi SM et. al., 2006), (Pandey A et. al., 2011), (Chandramouli C et. al., 2012), (Singh N et. al., 2006), analgesic (Chinnasamy RP et. al., 2010), (Mounika K et. al., 2010) antimicrobial (Venkatesh P, 2011) (Chaubey AK and Pandeya SN, 2012), anticonvulsant (Aboul-Fadl T et. al., 2003), antitubercular (Miri R et. al., 2013), anticancer (Ali SMM et. al., 2012), ( Wei D et. al., 2006), antioxidant (Avaji PG et. al., 2009), anthelmintic (Venugopala KN and Jayashree BS, 2003) and so forth. The nitrogen atom of azomethine may be involved in the formation of a hydrogen bond with the active centers of cell constituents and interferes in normal cell processes (Vashi K and Naik HB, 2004), (Li S et. al., 1996 ). Apart from biological activities, Schiff bases are also used as catalysts, intermediates in organic synthesis, dyes, pigments, polymer stabilizers and corrosion inhibitors (Chohan ZH et. al., 1997). Studies enlightened that metal complexes show greater biological activity than free organic compounds (Ershad S et. al., 2009). Augmentation of biological activity was reported by implementation of transition metals into Schiff bases (Tisato F et. al., 1994). Schiff bases played an influencing role in development of co-ordination chemistry and were involved as key point in the development of inorganic biochemistry and optical materials (Jarrahpour A et. al., 2007.) Schiff bases have been utilized as synthons in the preparation of a number of industrial and biologically active compounds like formazans, 4-thiazolidinines, benzoxazines, and so forth, via ring closure, cycloaddition and replacement reactions (Bhattacharya A et. al., 2003). Eg. Isatin Schiff base (3)
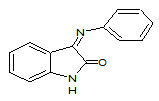
3
BIOLOGICAL ACTIVITIES OF QUINOXALINE DERIVATIVES
Antimicrobial Activities:
U. K. Singh et. al., reported the synthesis of Schiff’s and N-Mannich bases of isatin and its derivatives with 4-amino-N-carbamimidoyl benzene sulfonamide (4) and was tested for antibacterial activity by MIC method on strains: S. aureus, B. pumulis, B. subtilis, E. coli, S. abony, K. pneumoniae. All compounds exhibited very significant and better antibacterial activity (Singh UK et. al., 2010).
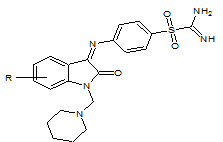
R = H, NO2, Cl, Br, CH3
4
Chhajed S.S et. al., reported the synthesis of schiff and mannich bases of isatin and its derivatives with quinoline (5). Investigation of antimicrobial activity of the compounds was made by the agar dilution method on strains: B. substilis, S. aureus, S. faecalis, E. Coli, P. aeruginosa, C. albicans A. niger. And the compounds are significantly active against bacteria and fungi (Chhajed SS and Padwal MS, 2010).
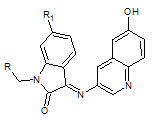
5
Aliasghar Jarrahpour et. al., reported the synthesis of some novel bis-schiff bases of isatin and their derivatives. These newly synthesized bis-schiff bases (6) were also tested for their antibacterial and antifungal activities by MIC method on strains: S. cerevisiae, S. aureus, C. albicans, E. coli (Aliasghar J et. al., 2007).
6
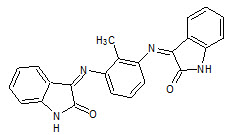
Ramachandran et. al., reported the synthesis of schiff and mannich bases of isatin derivatives (7) and was tested for antimicrobial activity by Cup-plate method on strains: like Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Klebsilla aerogenes, Candida albicans. Most of the compounds shown greater antibacterial and antifungal activities when compared with the standard drugs (Ramachandran S, 2011).
7
Seshaiah Krishnan Sridhar et. al., reported the synthesis of synthesis of hydrazones, schiff and mannich bases of isatin derivatives (8). The compounds were screened for antibacterial activity on strains: Bacillus subtillus, Staphylococcus aureous, E.coli and Pseudomonas aeruginosa. The minimum inhibitory concentrations of the active compounds were determined. 1- Diphenyl amino-methyl-3-(4-bromo phenylimino)-1, 3-dihydro-indol-3-one and 3-(4-bromo phenylimino)-5-nitro-1, 3-dihydroindol- 3-one were found to be the most active compounds of the series (Seshaiah KS et. al., 2001).
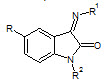
8
Sanjay Bari et. al., reported the synthesis and antimicrobial activity of some new isatin derivatives (9) antimicrobial activity of compounds with 5-bromo substitution showed the most favorable antimicrobial activity (Sanjay B et. al., 2006).
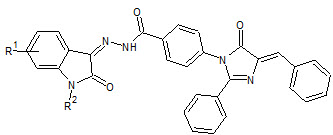
9
G. Sammaiah et. al., reported the synthesis of 2-aminobezoic acid (2-oxo-1, 2-dihydro-indol-3- ylidene)-hydrazides, as indole hydrazides have shown proven to be good antimicrobial agents. Some new series of indole hydrazides synthesized (10) few 2-amino benzoic acid (2-oxo-1, 2-dihydro-indol-3-ylidene)-hydrazides which showed good antimicrobial activity (Sammaiah G et. al., 2011).
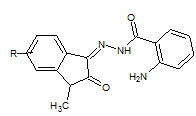
10
Antitubercular Activities
Sangamesh A. Patil et. al., reported the synthesis, biological evaluation Co (II), Ni (II),and Mn (II) metal complexes of novel isatin schiff base ligand (11) the complexes show activity against Mycobacterium tuberculosis strain H37Rv (Sangamesh AP, et. al., 2011)
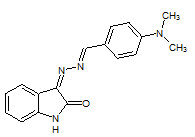
11
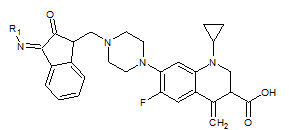
Sandeep K. Gupta et. al., reported the synthesis some thiobenzimidazolyl derivatives (12). Most of them reported good antitubercular activity against Mycobacterium tuberculosis (Sandeep KG and Shyam SP, 2011)
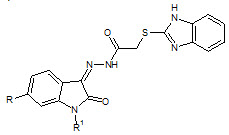
12
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE